Vancomycin Loaded Glycerol Monooleate Liquid Crystalline Phases Modified with Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Bulk LCPs
2.2.2. Characterization of LCPs
Polarized Light Microscopy (PLM)
Small Angle X-ray Scattering (SAXS)
In Vitro VHCl Release Test I
In Vitro VHCl Release Test II
High-Performance Liquid Chromatography (HPLC) Analysis of VHCl
3. Results
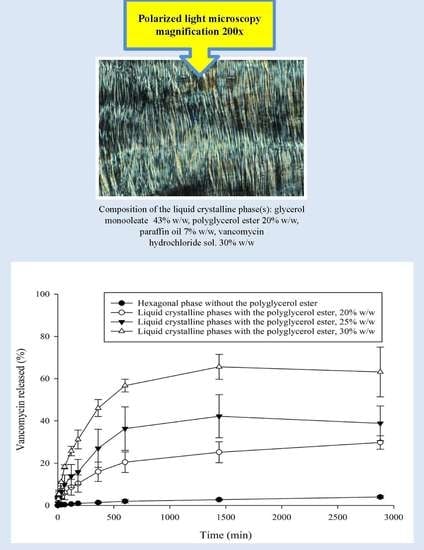
3.1. PLM
3.2. SAXS
3.3. In Vitro VHCl Release Test I
3.4. In Vitro VHCl Release Test II
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.; Fong, W.K.; Kirby, N.; Hanley, T.; Boyd, B.J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharm. 2011, 421, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.C.; Sadhale, Y.; Chilukuri, D.M. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001, 47, 229–250. [Google Scholar] [CrossRef]
- Clogston, J.; Caffrey, M. Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. J. Control. Release 2005, 107, 97–111. [Google Scholar] [CrossRef]
- Lee, K.W.Y.; Nguyen, T.H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef]
- Qiu, H.; Caffrey, M. The phase diagram of the monoolein/water system: Metastability and equilibrium aspects. Biomaterials 2000, 21, 223–234. [Google Scholar] [CrossRef]
- Rummel, G.; Hardmeyer, A.; Widmer, C.; Chiu, M.L.; Nollert, P.; Locher, K.P.; Pedruzzi, L.; Landau, E.M.; Rosenbusch, J.P. Lipidic Cubic Phases: New Matrices for the Three-Dimensional Crystallization of Membrane Proteins. J. Struct. Biol. 1998, 121, 82–91. [Google Scholar] [CrossRef]
- Imberg, A.; Evertsson, H.; Stilbs, P.; Kriechbaum, M.; Engström, S. On the self-assembly of monoolein in mixtures of water and a polar aprotic solvent. J. Phys. Chem. B 2003, 107, 2311–2318. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Mezzenga, R.; Leser, M.E. Investigating reversed liquid crystalline mesophases. Curr. Opin. Colloid Interface Sci. 2006, 11, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Hyde, S.T. Identification of Lyotropic Liquid Crystalline Mesophases. In Handbook of Applied Surface and Colloid Chemistry; Wiley-Blackwell: Hoboken, NJ, USA, 2001; pp. 299–332. [Google Scholar]
- Hyde, S.T. Bicontinuous structures in lyotropic liquid crystals and crystalline hyperbolic surfaces. Curr. Opin. Solid State Mater. Sci. 1996, 1, 653–662. [Google Scholar] [CrossRef]
- Zabara, A.; Mezzenga, R. Controlling molecular transport and sustained drug release in lipid-based liquid crystalline mesophases. J. Control. Release 2014, 188, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. PH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Bodmeier, R. Effect of dissolution media and additives on the drug release from cubic phase delivery systems. J. Control. Release 1997, 46, 215–222. [Google Scholar] [CrossRef]
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-assembled structures and pK a value of oleic acid in systems of biological levance. Langmuir 2010, 26, 11670–11679. [Google Scholar] [CrossRef]
- Fong, W.K.; Salentinig, S.; Prestidge, C.A.; Mezzenga, R.; Hawley, A.; Boyd, B.J. Generation of geometrically ordered lipid-based liquid-crystalline nanoparticles using biologically relevant enzymatic processing. Langmuir 2014, 30, 5373–5377. [Google Scholar] [CrossRef]
- Salentinig, S.; Tangso, K.J.; Hawley, A.; Boyd, B.J. pH-driven colloidal transformations based on the vasoactive drug nicergoline. Langmuir 2014, 30, 14776–14781. [Google Scholar] [CrossRef]
- Lindell, K.; Engblom, J.; Jonströmer, M.; Carlsson, A.; Engström, S. Influence of a charged phospholipid on the release pattern of timolol maleate from cubic liquid crystalline phases. Prog. Colloid Polym. Sci. 1998, 108, 111–118. [Google Scholar]
- Amar-Yuli, I.; Adamcik, J.; Blau, S.; Aserin, A.; Garti, N.; Mezzenga, R. Controlled embedment and release of DNA from lipidic reverse columnar hexagonal mesophases. Soft Matter 2011, 7, 8162–8168. [Google Scholar] [CrossRef]
- Kamo, T.; Nakano, M.; Kuroda, Y.; Handa, T. Effects of an amphipathic α-helical peptide on lateral pressure and water penetration in phosphatidylcholine and monoolein mixed membranes. J. Phys. Chem. B 2006, 110, 24987–24992. [Google Scholar] [CrossRef]
- Chemelli, A.; Conde-Valentín, B.; Uhlig, F.; Glatter, O. Amino Acid Induced Modification of Self-Assembled Monoglyceride-Based Nanostructures. Langmuir 2015, 31, 10377–10381. [Google Scholar] [CrossRef]
- Clogston, J.; Craciun, G.; Hart, D.J.; Caffrey, M. Controlling release from the lipidic cubic phase by selective alkylation. J. Control. Release 2005, 102, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Angelova, A.; Ollivon, M.; Bourgaux, C.; Campitelli, A. Diamond-type lipid cubic phase with large water channels. J. Am. Chem. Soc. 2003, 125, 7188–7189. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; De Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Control of the internal structure of MLO-based isasomes by the addition of diglycerol monooleate and soybean phosphatidylcholine. Langmuir 2006, 22, 9919–9927. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. Diffusion, molecular separation, and drug delivery from lipid mesophases with tunable water channels. Langmuir 2012, 28, 16455–16462. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Tyler, A.I.I.; McCarthy, N.L.C.; Parsons, E.S.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter 2015, 11, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Engström, S.; Lindahl, L.; Wallin, R.; Engblom, J. A study of polar lipid drug systems undergoing a thermoreversible lamellar-to-cubic phase transition. Int. J. Pharm. 1992, 86, 137–145. [Google Scholar] [CrossRef]
- Fong, W.K.; Hanley, T.; Boyd, B.J. Stimuli responsive liquid crystals provide “on-demand” drug delivery in vitro and in vivo. J. Control. Release 2009, 135, 218–226. [Google Scholar] [CrossRef]
- Fong, W.K.; Hanley, T.L.; Thierry, B.; Kirby, N.; Boyd, B.J. Plasmonic nanorods provide reversible control over nanostructure of self-assembled drug delivery materials. Langmuir 2010, 26, 6136–6139. [Google Scholar] [CrossRef]
- Fong, W.K.; Hanley, T.L.; Thierry, B.; Kirby, N.; Waddington, L.J.; Boyd, B.J. Controlling the nanostructure of gold nanorod-lyotropic liquid-crystalline hybrid materials using near-infrared laser irradiation. Langmuir 2012, 28, 14450–14460. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Handschin, S.; Bolisetty, S.; Mezzenga, R. Twofold light and magnetic responsive behavior in nanoparticle-lyotropic liquid crystal systems. Langmuir 2012, 28, 5589–5595. [Google Scholar] [CrossRef]
- Fong, W.K.; Malic, N.; Evans, R.A.; Hawley, A.; Boyd, B.J.; Hanley, T.L. Alkylation of spiropyran moiety provides reversible photo-control over nanostructured soft materials. Biointerphases 2012, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangso, K.J.; Fong, W.K.; Darwish, T.; Kirby, N.; Boyd, B.J.; Hanley, T.L. Novel spiropyran amphiphiles and their application as light-responsive liquid crystalline components. J. Phys. Chem. B 2013, 117, 10203–10210. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Paasonen, L.; Yliperttula, M.; Urtti, A.; Rappolt, M. Structural elucidation of light activated vesicles. J. Phys. Chem. Lett. 2010, 1, 962–966. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Bolisetty, S.; Mezzenga, R. Macroscopic alignment of lyotropic liquid crystals using magnetic nanoparticles. Adv. Mater. 2011, 23, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Vallooran, J.J.; Negrini, R.; Mezzenga, R. Controlling anisotropic drug diffusion in lipid-Fe3O4 nanoparticle hybrid mesophases by magnetic alignment. Langmuir 2013, 29, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Milak, S.; Chemelli, A.; Glatter, O.; Zimmer, A. Vancomycin ocular delivery systems based on glycerol monooleate reversed hexagonal and reversed cubic liquid crystalline phases. Eur. J. Pharm. Biopharm. 2019, 139, 279–290. [Google Scholar] [CrossRef]
- Müller-Goymann, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm. 2004, 58, 343–356. [Google Scholar] [CrossRef]
- Gonjari, I.D.; Hosmani, A.H.; Karmarkar, A.B.; Godage, A.S.; Kadam, S.B.; Dhabale, P.N. Formulation and evaluation of in situ gelling thermoreversible mucoadhesive gel of fluconazole. Drug Discov. Ther. 2009, 3, 6–9. [Google Scholar]
- Higuchi, W.I. Diffusional Models Useful in Biopharmaceutics. J. Pharm. Sci. 1967, 56, 315–324. [Google Scholar] [CrossRef]
- Kwon, T.K.; Kim, J.C. Complex coacervation-controlled release from monoolein cubic phase containing silk fibroin and alginate. Biomacromolecules 2011, 12, 466–471. [Google Scholar] [CrossRef]
- Schwartz, J.B.; Simonelli, A.P.; Higuchi, W.I. Drug Release from Wax Matrices, I. J. Pharm. Sci. 2017, 57, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Libster, D.; Aserin, A.; Yariv, D.; Shoham, G.; Garti, N. Concentration- and temperature-induced effects of incorporated desmopressin on the properties of reverse hexagonal mesophase. J. Phys. Chem. B 2009, 113, 6336–6346. [Google Scholar] [CrossRef] [PubMed]
- Amar-Yuli, I.; Wachtel, E.; Shoshan, E.B.; Danino, D.; Aserin, A.; Garti, N. Hexosome and hexagonal phases mediated by hydration and polymeric stabilizer. Langmuir 2007, 23, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Da Dong, Y.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and dispersed aqueous phase behavior of phytantriol: Effect of vitamin E acetate and F127 polymer on liquid crystal nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef]
- Bisset, N.B.; Boyd, B.J.; Da Dong, Y. Tailoring liquid crystalline lipid nanomaterials for controlled release of macromolecules. Int. J. Pharm. 2015, 495, 241–248. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.U.; Shin, W.C.; Lee, H.Y.; Kim, J.D.; Kim, Y.C.; Tae, G.; Lee, K.; Lee, S.J.; Kim, J. Monoolein cubic phases containing hydrogen peroxide. Colloids Surf. B Biointerfaces 2004, 36, 161–166. [Google Scholar] [CrossRef]
- Chang, C.M.; Bodmeier, R. Binding of drugs to monoglyceride-based drug delivery systems. Int. J. Pharm. 1997, 147, 135–142. [Google Scholar] [CrossRef]
- Wyatt, D.; Dorschel, D. A cubic-phase delivery system composed of glyceryl monooleate and water for sustained release of water-soluble drugs. Pharm. Technol. 1992, 16, 116–130. [Google Scholar]
- Chang, C.M.; Bodmeier, R. Low viscosity monoglyceride-based drug delivery systems transforming into a highly viscous cubic phase. Int. J. Pharm. 1998, 173, 51–60. [Google Scholar] [CrossRef]
- Chemelli, A.; Maurer, M.; Geier, R.; Glatter, O. Optimized loading and sustained release of hydrophilic proteins from internally nanostructured particles. Langmuir 2012, 28, 16788–16797. [Google Scholar] [CrossRef]
- Sautou-Miranda, V.; Libert, F.; Grand-Boyer, A.; Gellis, C.; Chopineau, J. Impact of deep freezing on the stability of 25 mg/mL vancomycin ophthalmic solutions. Int. J. Pharm. 2002, 234, 205–212. [Google Scholar] [CrossRef]
- Kinnunen, H.; Sharma, V.K.; Contrerasrojas, L.R.; Yu, Y.; Alleman, C.; Sreedhara, A.; Fischer, S.; Khawli, L.A.; Yohe, S.; Bumbaca, D.; et al. A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components. J. Control. Release 2015, 214, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnunen, H.M.; Mrsny, R.J. Improving the outcomes of biopharmaceutical delivery via the subcutaneous route by understanding the chemical, physical and physiological properties of the subcutaneous injection site. J. Control. Release 2014, 182, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stylianopoulos, T.; Poh, M.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Ferencz, J.R.; Assia, E.I.; Diamantstein, L.; Rubinstein, E. Vancomycin concentration in the vitreous after intravenous and intravitreal administration for postoperative endophthalmitis. Arch. Ophthalmol. 1999, 117, 1023–1027. [Google Scholar] [CrossRef] [Green Version]





| Hexagonal Phase without Tuning Agent | % Glycerol Monooleate | Structure Parameter * (nm) | Radius of Water Channels in The Hexagonal Phase (Calculated) (nm) | ||
|---|---|---|---|---|---|
| Hexagonal Phase without tuning Agent [37] | HII | Lα | Ia3d | HII | |
| 63 | 6538 ± 0.02(37) | / | / | 3.70 | |
| LCP tuned with | structure parameter * (nm) | radius of water channels in the hexagonal phase (calculated) (nm) | |||
| PE% (w/w) | HII | Lα | Ia3d | HII | |
| 10 | 53 | 7.253 ± 0.06 | (4.965 ± 0.05) | 12.1387 ± 0.05 | 4.63 |
| 20 | 43 | 7.625 ± 0.07 | 4.887 ± 0.01 | ND | 5.36 |
| 25 | 38 | 7.996 ± 0.07 | 4.913 ± 0.01 | ND | 5.86 |
| 30 | 33 | 8.700 ± 0.20 | 5.009 ± 0.04 | ND | 6.63 |
| TC% (w/w) | HII | Lα | / | HII | |
| 1 | 63 | 6.732 ± 0.02 | ND ** | / | 3.81 |
| 5 | 63 | 6.379 ± 0.06 | ND ** | / | 3.61 |
| 10 | 63 | 6.061 ± 0.04 | ND ** | / | 3.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milak, S.; Chemelli, A.; Glatter, O.; Zimmer, A. Vancomycin Loaded Glycerol Monooleate Liquid Crystalline Phases Modified with Surfactants. Pharmaceutics 2020, 12, 521. https://doi.org/10.3390/pharmaceutics12060521
Milak S, Chemelli A, Glatter O, Zimmer A. Vancomycin Loaded Glycerol Monooleate Liquid Crystalline Phases Modified with Surfactants. Pharmaceutics. 2020; 12(6):521. https://doi.org/10.3390/pharmaceutics12060521
Chicago/Turabian StyleMilak, Spomenka, Angela Chemelli, Otto Glatter, and Andreas Zimmer. 2020. "Vancomycin Loaded Glycerol Monooleate Liquid Crystalline Phases Modified with Surfactants" Pharmaceutics 12, no. 6: 521. https://doi.org/10.3390/pharmaceutics12060521