Adjuvant Drug-Assisted Bone Healing: Advances and Challenges in Drug Delivery Approaches
Abstract
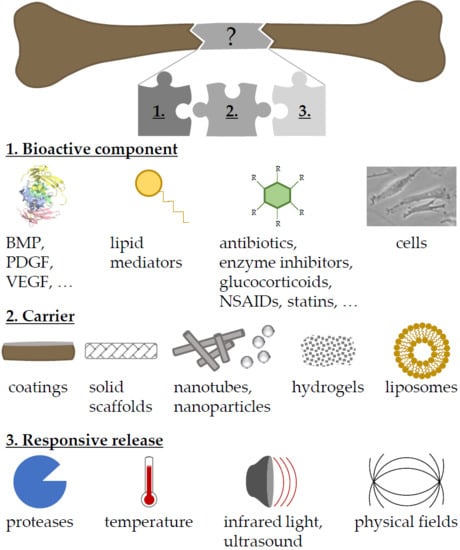
:1. Introduction
2. Carriers for Drug Delivery
2.1. Composition of Scaffolds
2.1.1. Natural Polymers
2.1.2. Calcium Phosphates
2.1.3. Synthetic Polymers
2.1.4. Hybrid Scaffolds
2.2. Scaffold Formulations
2.2.1. Drug-Releasing Coatings
2.2.2. Hydrogels
2.2.3. Nanotubes and Nanofibers
2.2.4. Particles
2.2.5. Liposomes and Micelles
3. Dual Drug Delivery
3.1. Growth Factors
3.2. Growth Factors and Bisphosphonates
3.3. Growth Factors and Enzyme Inhibitors or Receptor Agonists
3.4. Growth Factors and Antibiotics
3.5. Growth Factors and Cells
4. Triggered Drug Delivery
4.1. Proteolytic Enzymes
4.2. Redox Environment
4.3. pH Alteration
4.4. Temperature
4.5. Near-Infrared Light Irradiation
4.6. Physical Fields
4.7. Ultrasound
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro, O.; Linero, I. Regenerative Medicine: A New Paradigm in Bone Regeneration. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; InTech: Rijeka, Croatia, 2016; pp. 253–274. [Google Scholar]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug. Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammelt, S. Management of ankle fractures in the elderly. Efort Open Rev. 2016, 1, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Fritzsche, H.; Hofbauer, C.; Schaser, K.D. Malignant tumours of the foot and ankle. Foot Ankle Surg. 2019, in press. [Google Scholar] [CrossRef]
- Wang, Y.; Newman, M.R.; Benoit, D.S.W. Development of controlled drug delivery systems for bone fracture-targeted therapeutic delivery: A review. Eur. J. Pharm. Biopharm. 2018, 127, 223–236. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, H.; Vakili-Ghartavol, R. Bone Regeneration: Current Status and Future Prospects. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; InTech: Rijeka, Croatia, 2016; pp. 3–25. [Google Scholar]
- Loi, F.; Cordova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Seo, S.J.; Won, J.E.; Lee, E.J.; Jang, J.H.; Knowles, J.C.; Kim, H.W. Therapeutically relevant aspects in bone repair and regeneration. Mater. Today 2015, 18, 573–589. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheirallah, M.; Almeshaly, H. Present Strategies for Critical Bone Defects Regeneration. Oral Health Case Rep. 2016, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, 3–6. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Lima, A.C.; Ferreira, H.; Reis, R.L.; Neves, N.M. Biodegradable polymers: An update on drug delivery in bone and cartilage diseases. Expert Opin. Drug. Deliv. 2019, 16, 795–813. [Google Scholar] [CrossRef]
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of nanomaterials for bone-targeted drug delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef]
- Rothe, R.; Schulze, S.; Neuber, C.; Hauser, S.; Rammelt, S.; Pietzsch, J. Adjuvant drug-assisted bone healing: Part I - Modulation of inflammation. Clin. Hemorheol. Microcirc. 2019, 73, 381–408. [Google Scholar] [CrossRef]
- Rothe, R.; Schulze, S.; Neuber, C.; Hauser, S.; Rammelt, S.; Pietzsch, J. Adjuvant drug-assisted bone healing: Part II—Modulation of angiogenesis. Clin. Hemorheol. Microcirc. 2019, 73, 409–438. [Google Scholar] [CrossRef]
- Rothe, R.; Schulze, S.; Neuber, C.; Hauser, S.; Rammelt, S.; Pietzsch, J. Adjuvant drug-assisted bone healing: Part III—Further strategies for local and systemic modulation. Clin. Hemorheol. Microcirc. 2019, 73, 439–488. [Google Scholar] [CrossRef]
- Dang, L.; Liu, J.; Li, F.; Wang, L.; Li, D.; Guo, B.; He, X.; Jiang, F.; Liang, C.; Liu, B. Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders. Int. J. Mol. Sci. 2016, 17, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, K.; Aw, M.S.; Findlay, D.; Losic, D. Local drug delivery to the bone by drug-releasing implants: Perspectives of nano-engineered titania nanotube arrays. Deliv 2012, 3, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1504. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; D’Asta, F.; Brandi, M.L. Drug delivery using composite scaffolds in the context of bone tissue engineering. Clin. Cases Min. Bone Metab. 2013, 10, 155. [Google Scholar]
- Briquez, P.S.; Clegg, L.E.; Martino, M.M.; Mac Gabhann, F.; Hubbell, J.A. Design principles for therapeutic angiogenic materials. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Ferracini, R.; Martinez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [Green Version]
- Sokolsky-Papkov, M.; Agashi, K.; Olaye, A.; Shakesheff, K.; Domb, A.J. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 187–206. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alencastre, I.S.; Sousa, D.M.; Alves, C.J.; Leitao, L.; Neto, E.; Aguiar, P.; Lamghari, M. Delivery of Pharmaceutics to Bone: Nanotechnologies, High-Throughput Processing and in Silico Mathematical Models. Eur. Cell Mater. 2016, 31, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kyllonen, L.; D’Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, J.A.; Sato, M.R.; Scardueli, C.R.; Lopes de Oliveira, G.J.P.; Abucafy, M.P.; Chorilli, M. Bioactive Molecule-loaded Drug Delivery Systems to Optimize Bone Tissue Repair. Curr. Protein Pept. Sci. 2017, 18, 850–863. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, H.W. Core-shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 2015, 21, 2–19. [Google Scholar] [CrossRef]
- Kheirallah, M.; Almeshaly, H. Bone Graft Substitutes for Bone Defect Regeneration. A Collective Review. IJDOS 2016, 3, 247–255. [Google Scholar]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Borner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. Am. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhang, Q.; Li, J.; Liu, Y.; Hou, Z.; Chen, W.; Jin, L.; Tian, Y.; Ju, L.; Liu, B.; et al. Local application of an ibandronate/collagen sponge improves femoral fracture healing in ovariectomized rats. PLoS ONE 2017, 12, e0187683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regi, M.; Izquierdo-Barba, I.; Colilla, M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos. Trans. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.; Al-Sulaimani, A.F.; Beeran, A.E.; Chalisserry, E.P.; Varma, H.P.R.; Al Amri, M.D. Drug Delivery Systems in Bone Regeneration and Implant Dentistry. In Current Concepts in Dental Implantology; Turkyilmaz, I., Ed.; InTech: Rijeka, Croatia, 2015; pp. 239–265. [Google Scholar]
- Mourino, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface. 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.A. The Silica-based Formulations for Drug Delivery, Bone Treatment, and Bone Regeneration. Chembioeng. Rev. 2016, 3, 229–246. [Google Scholar] [CrossRef]
- Rammelt, S.; Neumann, M.; Hanisch, U.; Reinstorf, A.; Pompe, W.; Zwipp, H.; Biewener, A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J. Biomed. Mater. Res. A 2005, 73, 284–294. [Google Scholar] [CrossRef]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Poldervaart, M.T.; Wang, H.; van der Stok, J.; Weinans, H.; Leeuwenburgh, S.C.; Oner, F.C.; Dhert, W.J.; Alblas, J. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS ONE 2013, 8, e72610. [Google Scholar] [CrossRef]
- Chu, T.M.; Warden, S.J.; Turner, C.H.; Stewart, R.L. Segmental bone regeneration using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials 2007, 28, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Maehara, H.; Sotome, S.; Yoshii, T.; Torigoe, I.; Kawasaki, Y.; Sugata, Y.; Yuasa, M.; Hirano, M.; Mochizuki, N.; Kikuchi, M.; et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J. Orthop. Res. 2010, 28, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Tanaka, T.; Chazono, M.; Kikuchi, T. Repair of segmental bone defects in rabbit tibiae using a complex of beta-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials 2006, 27, 5118–5126. [Google Scholar] [CrossRef] [PubMed]
- Al-Zube, L.; Breitbart, E.A.; O’Connor, J.P.; Parsons, J.R.; Bradica, G.; Hart, C.E.; Lin, S.S. Recombinant human platelet-derived growth factor BB (rhPDGF-BB) and beta-tricalcium phosphate/collagen matrix enhance fracture healing in a diabetic rat model. J. Orthop. Res. 2009, 27, 1074–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernike, E.; Montjovent, M.O.; Liu, Y.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W.; Klenke, F.M. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cell Mater. 2010, 19, 30–40. [Google Scholar] [PubMed]
- Huang, X.; Huang, Z.; Li, W. Highly efficient release of simvastatin from simvastatin-loaded calcium sulphate scaffolds enhances segmental bone regeneration in rabbits. Mol. Med. Rep. 2014, 9, 2152–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyan, M.; Sato, D.; Oda, M.; Machida, T.; Kobayashi, H.; Nakamura, T.; Kasugai, S. Bone formation with the combination of simvastatin and calcium sulfate in critical-sized rat calvarial defect. J. Pharm. Sci. 2007, 104, 384–386. [Google Scholar] [CrossRef] [Green Version]
- Khurana, K.; Guillem-Marti, J.; Soldera, F.; Mucklich, F.; Canal, C.; Ginebra, M.P. Injectable calcium phosphate foams for the delivery of Pitavastatin as osteogenic and angiogenic agent. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 760–770. [Google Scholar] [CrossRef]
- Mendoza, S.; Noa, M.; Mas, R.; Mendoza, N. Comparison of the effects of D-003, a mixture of high-molecular-weight aliphatic acids from sugarcane wax, and pravastatin on bones and osteoclast apoptosis of ovariectomized rats. Drugs Exp. Clin. Res. 2005, 31, 181–191. [Google Scholar]
- Shah, S.R.; Werlang, C.A.; Kasper, F.K.; Mikos, A.G. Novel applications of statins for bone regeneration. Natl. Sci. Rev. 2015, 2, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, W.; Pfützner, A.; Müller, J.; Pietzsch, J.; Forst, T.; Karagiannis, E.; Lübben, G.; Hanefeld, M. Effects of pioglitazone and/or simvastatin on low density lipoprotein subfractions in non-diabetic patients with high cardiovascular risk: A sub-analysis from the PIOSTAT study. Atherosclerosis 2008, 201, 155–162. [Google Scholar] [CrossRef]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Yun, Y.P.; Kim, S.E.; Song, H.R. The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model. Int. J. Mol. Sci. 2015, 16, 26738–26753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baier, M.; Staudt, P.; Klein, R.; Sommer, U.; Wenz, R.; Grafe, I.; Meeder, P.J.; Nawroth, P.P.; Kasperk, C. Strontium enhances osseointegration of calcium phosphate cement: A histomorphometric pilot study in ovariectomized rats. J. Orthop. Surg. Res. 2013, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thormann, U.; Ray, S.; Sommer, U.; Elkhassawna, T.; Rehling, T.; Hundgeburth, M.; Henss, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.; Zhou, W.; Jiang, Y.; Wu, X.; Xu, Z.; Yang, M.; Xie, J. Effects of strontium-modified calcium phosphate cement combined with bone morphogenetic protein-2 on osteoporotic bone defects healing in rats. J. Biomater. Appl. 2018, 33, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium (II) and mechanical loading additively augment bone formation in calcium phosphate scaffolds. J. Orthop. Res. 2018, 36, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Rentsch, C.; Schneiders, W.; Hess, R.; Rentsch, B.; Bernhardt, R.; Spekl, K.; Schneider, K.; Scharnweber, D.; Biewener, A.; Rammelt, S. Healing properties of surface-coated polycaprolactone-co-lactide scaffolds: A pilot study in sheep. J. Biomater. Appl. 2014, 28, 654–666. [Google Scholar] [CrossRef] [Green Version]
- Neuber, C.; Schulze, S.; Forster, Y.; Hofheinz, F.; Wodke, J.; Moller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; Rammelt, S.; et al. Biomaterials in repairing rat femoral defects: In vivo insights from small animal positron emission tomography/computed tomography (PET/CT) studies. Clin. Hemorheol. Microcirc. 2019, 73, 177–194. [Google Scholar] [CrossRef]
- Li, M.; Ke, H.Z.; Qi, H.; Healy, D.R.; Li, Y.; Crawford, D.T.; Paralkar, V.M.; Owen, T.A.; Cameron, K.O.; Lefker, B.A.; et al. A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J. Bone Min. Res. 2003, 18, 2033–2042. [Google Scholar] [CrossRef]
- Yoshii, T.; Hafeman, A.E.; Nyman, J.S.; Esparza, J.M.; Shinomiya, K.; Spengler, D.M.; Mundy, G.R.; Gutierrez, G.E.; Guelcher, S.A. A sustained release of lovastatin from biodegradable, elastomeric polyurethane scaffolds for enhanced bone regeneration. Tiss. Eng. Part A 2010, 16, 2369–2379. [Google Scholar] [CrossRef]
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Min. Res. 2006, 21, 735–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Moriyama, Y.; Ayukawa, Y.; Rakhmatia, Y.D.; Tomita, Y.; Yasunami, N.; Koyano, K. Generation and histomorphometric evaluation of a novel fluvastatin-containing poly(lactic-co-glycolic acid) membrane for guided bone regeneration. Odontology 2019, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Koh, A.J.; Danciu, T.; McCauley, L.K.; Ma, P.X. Preprogrammed Long-Term Systemic Pulsatile Delivery of Parathyroid Hormone to Strengthen Bone. Adv. Healthc. Mater. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, M.; Koh, A.J.; Jin, X.; McCauley, L.K.; Ma, P.X. Local pulsatile PTH delivery regenerates bone defects via enhanced bone remodeling in a cell-free scaffold. Biomaterials 2017, 114, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, O.; Song, S.J.; Kang, S.W.; Putnam, A.J.; Kim, B.S. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials 2007, 28, 2763–2771. [Google Scholar] [CrossRef]
- Kim, T.H.; Yun, Y.P.; Park, Y.E.; Lee, S.H.; Yong, W.; Kundu, J.; Jung, J.W.; Shim, J.H.; Cho, D.W.; Kim, S.E.; et al. In vitro and in vivo evaluation of bone formation using solid freeform fabrication-based bone morphogenic protein-2 releasing PCL/PLGA scaffolds. Biomed. Mater. 2014, 9, 025008. [Google Scholar] [CrossRef]
- Hoshino, M.; Egi, T.; Terai, H.; Namikawa, T.; Kato, M.; Hashimoto, Y.; Takaoka, K. Repair of long intercalated rib defects in dogs using recombinant human bone morphogenetic protein-2 delivered by a synthetic polymer and beta-tricalcium phosphate. J. Biomed. Mater. Res. A 2009, 90, 514–521. [Google Scholar] [CrossRef]
- Kokubo, S.; Fujimoto, R.; Yokota, S.; Fukushima, S.; Nozaki, K.; Takahashi, K.; Miyata, K. Bone regeneration by recombinant human bone morphogenetic protein-2 and a novel biodegradable carrier in a rabbit ulnar defect model. Biomaterials 2003, 24, 1643–1651. [Google Scholar] [CrossRef]
- Keskin, D.S.; Tezcaner, A.; Korkusuz, P.; Korkusuz, F.; Hasirci, V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials 2005, 26, 4023–4034. [Google Scholar] [CrossRef]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra93. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, H.E. Accelerated bony defect healing by chitosan/silica hybrid membrane with localized bone morphogenetic protein-2 delivery. Mater Sci Eng C Mater Biol Appl 2016, 59, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Piskin, E.; Isoglu, I.A.; Bolgen, N.; Vargel, I.; Griffiths, S.; Cavusoglu, T.; Korkusuz, P.; Guzel, E.; Cartmell, S. In vivo performance of simvastatin-loaded electrospun spiral-wound polycaprolactone scaffolds in reconstruction of cranial bone defects in the rat model. J. Biomed. Mater. Res. A 2009, 90, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.K.; Fahmy, R.H. Localized rosuvastatin via implantable bioerodible sponge and its potential role in augmenting bone healing and regeneration. Drug Deliv 2016, 23, 3181–3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monjo, M.; Rubert, M.; Wohlfahrt, J.C.; Ronold, H.J.; Ellingsen, J.E.; Lyngstadaas, S.P. In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 2010, 6, 1405–1412. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 184–194. [Google Scholar] [CrossRef]
- Odekerken, J.C.; Welting, T.J.; Arts, J.J.; Walenkamp, G.H.; Emans, P.J. Modern orthopaedic implant coatings—Their pro’s, con’s and evaluation methods. In Modern Surface Engineering Treatments; Aliofkhazraei, M., Ed.; InTech: Rijeka, Croatia, 2013; pp. 45–73. pp. 45–73. [Google Scholar]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Natural and Genetically Engineered Proteins for Tissue Engineering. Prog. Polym. Sci. 2012, 37, 1–17. [Google Scholar] [CrossRef]
- Petrie Aronin, C.E.; Shin, S.J.; Naden, K.B.; Rios, P.D., Jr.; Sefcik, L.S.; Zawodny, S.R.; Bagayoko, N.D.; Cui, Q.; Khan, Y.; Botchwey, E.A. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials 2010, 31, 6417–6424. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Segar, C.E.; Chu, Y.; Wang, T.W.; Lin, Y.; Yang, C.; Du, X.; Ogle, R.C.; Cui, Q.; Botchwey, E.A. Bioactive lipid coating of bone allografts directs engraftment and fate determination of bone marrow-derived cells in rat GFP chimeras. Biomaterials 2015, 64, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Krieger, J.; Huang, C.; Das, A.; Francis, M.P.; Ogle, R.; Botchwey, E. Enhanced osseous integration of human trabecular allografts following surface modification with bioactive lipids. Drug Deliv. Transl. Res. 2016, 6, 96–104. [Google Scholar] [CrossRef]
- Huang, C.; Das, A.; Barker, D.; Tholpady, S.; Wang, T.; Cui, Q.; Ogle, R.; Botchwey, E. Local delivery of FTY720 accelerates cranial allograft incorporation and bone formation. Cell Tissue Res. 2012, 347, 553–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone regeneration in critical bone defects using three-dimensionally printed beta-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassbender, M.; Minkwitz, S.; Strobel, C.; Schmidmaier, G.; Wildemann, B. Stimulation of bone healing by sustained bone morphogenetic protein 2 (BMP-2) delivery. Int. J. Mol. Sci. 2014, 15, 8539–8552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildemann, B.; Lange, K.; Strobel, C.; Fassbender, M.; Willie, B.; Schmidmaier, G. Local BMP-2 application can rescue the delayed osteotomy healing in a rat model. Injury 2011, 42, 746–752. [Google Scholar] [CrossRef]
- Shah, N.J.; Hyder, M.N.; Moskowitz, J.S.; Quadir, M.A.; Morton, S.W.; Seeherman, H.J.; Padera, R.F.; Spector, M.; Hammond, P.T. Surface-mediated bone tissue morphogenesis from tunable nanolayered implant coatings. Sci Transl Med 2013, 5, 191ra83. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Pajarinen, J.; Lu, L.; Nabeshima, A.; Cordova, L.A.; Yao, Z.; Goodman, S.B. NF-kappaB as a Therapeutic Target in Inflammatory-Associated Bone Diseases. Adv. Protein Chem. Struct. Biol. 2017, 107, 117–154. [Google Scholar]
- He, J.; Huang, T.; Gan, L.; Zhou, Z.; Jiang, B.; Wu, Y.; Wu, F.; Gu, Z. Collagen-infiltrated porous hydroxyapatite coating and its osteogenic properties: In vitro and in vivo study. J. Biomed. Mater. Res. A 2012, 100, 1706–1715. [Google Scholar] [CrossRef]
- Pauly, S.; Luttosch, F.; Morawski, M.; Haas, N.P.; Schmidmaier, G.; Wildemann, B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone 2009, 45, 505–511. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhu, S.; Luo, E.; Li, J.; Feng, G.; Liao, Y.; Hu, J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials 2010, 31, 9006–9014. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, S.; Liu, X.; Bao, C.; Hu, J. The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials 2009, 30, 1790–1796. [Google Scholar] [CrossRef]
- Greiner, S.H.; Wildemann, B.; Back, D.A.; Alidoust, M.; Schwabe, P.; Haas, N.P.; Schmidmaier, G. Local application of zoledronic acid incorporated in a poly(D,L-lactide)-coated implant accelerates fracture healing in rats. Acta Orthop. 2008, 79, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Pioletti, D.P.; Laib, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbuz, D.S.; Hu, Y.; Kim, W.Y.; Duan, K.; Masri, B.A.; Oxland, T.R.; Burt, H.; Wang, R.; Duncan, C.P. Enhanced gap filling and osteoconduction associated with alendronate-calcium phosphate-coated porous tantalum. J. Bone Jt. Surg. Am. 2008, 90, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Gauthier, O.; Laib, S.; Bujoli, B.; Guicheux, J.; Janvier, P.; van Lenthe, G.H.; Muller, R.; Zambelli, P.Y.; Bouler, J.M.; et al. Local delivery of bisphosphonate from coated orthopedic implants increases implants mechanical stability in osteoporotic rats. J. Biomed. Mater. Res. A 2006, 76, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiwara, H.; Yamaza, T.; Yoshinari, M.; Goto, T.; Iyama, S.; Atsuta, I.; Kido, M.A.; Tanaka, T. The bisphosphonate pamidronate on the surface of titanium stimulates bone formation around tibial implants in rats. Biomaterials 2005, 26, 581–587. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: An in vitro and in vivo study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef]
- Gibbs, D.M.; Black, C.R.; Dawson, J.I.; Oreffo, R.O. A review of hydrogel use in fracture healing and bone regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 187–198. [Google Scholar] [CrossRef]
- Tabata, Y. Current status of regenerative medical therapy based on drug delivery technology. Reprod. Biomed. Online 2008, 16, 70–80. [Google Scholar] [CrossRef]
- Lienemann, P.S.; Lutolf, M.P.; Ehrbar, M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1078–1089. [Google Scholar] [CrossRef]
- Fukui, T.; Ii, M.; Shoji, T.; Matsumoto, T.; Mifune, Y.; Kawakami, Y.; Akimaru, H.; Kawamoto, A.; Kuroda, T.; Saito, T.; et al. Therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J. Bone Min. Res. 2012, 27, 1118–1131. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, L.Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. A 2015, 103, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Yang, D.H.; Lee, J.B.; Heo, D.N.; Kwon, Y.D.; Youn, I.C.; Choi, K.; Hong, J.H.; Kim, G.T.; Choi, Y.S.; et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials 2011, 32, 8161–8171. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Nomoto, H.; Okumori, N.; Miura, T.; Yoshinari, M. Osteogenic effect of fluvastatin combined with biodegradable gelatin-hydrogel. Dent. Mater. J. 2012, 31, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.J.; Jingushi, S.; Aoyama, I.; Anzai, J.; Hirata, G.; Tamura, M.; Iwamoto, Y. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. J. Bone Min. Metab. 2004, 22, 303–309. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Oka, H.; Jingushi, S.; Izumi, T.; Fukunaga, M.; Sato, K.; Matsushita, T.; Nakamura, K. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J. Bone Min. Res. 2010, 25, 2735–2743. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006, 12, 1305–1311. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Diab, T.; Pritchard, E.M.; Uhrig, B.A.; Boerckel, J.D.; Kaplan, D.L.; Guldberg, R.E. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. J. Mech. Behav. Biomed. Mater. 2012, 11, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Webster, T.J. Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin. Drug Deliv. 2009, 6, 851–864. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, S.J.; Wikesjo, U.M.E.; Johansson, P.H.; Johansson, C.B.; Sul, Y.T. Bone tissue response following local drug delivery of bisphosphonate through titanium oxide nanotube implants in a rabbit model. J. Clin. Periodontol. 2017, 44, 941–949. [Google Scholar] [CrossRef]
- Shen, X.K.; Ma, P.P.; Hu, Y.; Xu, G.Q.; Xu, K.; Chen, W.Z.; Ran, Q.C.; Dai, L.L.; Yu, Y.L.; Mu, C.Y.; et al. Alendronate-loaded hydroxyapatite-TiO2 nanotubes for improved bone formation in osteoporotic rabbits. J. Mater. Chem. B 2016, 4, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Huang, B.J.; Kaltz, S.R.; Sur, S.; Newcomb, C.J.; Stock, S.R.; Shah, R.N.; Stupp, S.I. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 2013, 34, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Jin, Q.; Giannobile, W.V.; Ma, P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007, 28, 2087–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Shiota, M.; Fujii, M.; Chen, K.; Shimogishi, M.; Sato, M.; Kasugai, S. Combination of simvastatin and hydroxyapatite fiber induces bone augmentation. Open J Regen Med 2013, 02, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; et al. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srouji, S.; Ben-David, D.; Lotan, R.; Livne, E.; Avrahami, R.; Zussman, E. Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: In vitro and in vivo evaluation. Tissue Eng. Part A 2011, 17, 269–277. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kolambkar, Y.M.; Boerckel, J.D.; Dupont, K.M.; Bajin, M.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone 2011, 49, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.C.; Nie, H.; Ho, M.L.; Wang, C.K.; Wang, C.H. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol. Bioeng. 2008, 99, 996–1006. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, D.; Zhou, Y.; Wang, C.; Gao, M.; Jiang, H.; Wang, H. Biological activity of a nanofibrous barrier membrane containing bone morphogenetic protein formed by core-shell electrospinning as a sustained delivery vehicle. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 541–552. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; Garcia, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for bone tissue engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Werkmeister, J.A.; Wang, J.; Glattauer, V.; McLean, K.M.; Liu, C. Bone regeneration using photocrosslinked hydrogel incorporating rhBMP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticles. Biomaterials 2014, 35, 2730–2742. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, J.; Xia, Y.; Yuan, Y.; Zhang, H.; Xu, S.; Lin, K. Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration. Int. J. Nanomed. 2018, 13, 4083–4092. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Yoshii, T.; Hafeman, A.E.; Nyman, J.S.; Wenke, J.C.; Guelcher, S.A. The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora. Biomaterials 2009, 30, 6768–6779. [Google Scholar] [CrossRef]
- Rahman, C.V.; Ben-David, D.; Dhillon, A.; Kuhn, G.; Gould, T.W.; Muller, R.; Rose, F.R.; Shakesheff, K.M.; Livne, E. Controlled release of BMP-2 from a sintered polymer scaffold enhances bone repair in a mouse calvarial defect model. J. Tissue Eng. Regen. Med. 2014, 8, 59–66. [Google Scholar] [CrossRef]
- Chung, Y.I.; Ahn, K.M.; Jeon, S.H.; Lee, S.Y.; Lee, J.H.; Tae, G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J. Control Release 2007, 121, 91–99. [Google Scholar] [CrossRef]
- Henslee, A.M.; Spicer, P.P.; Yoon, D.M.; Nair, M.B.; Meretoja, V.V.; Witherel, K.E.; Jansen, J.A.; Mikos, A.G.; Kasper, F.K. Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 2011, 3627–3637. [Google Scholar] [CrossRef] [Green Version]
- Link, D.P.; van den Dolder, J.; van den Beucken, J.J.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-beta 1 loaded gelatin microparticles. Biomaterials 2008, 29, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kim, K.H.; Shin, S.Y.; Rhyu, I.C.; Lee, Y.M.; Park, Y.J.; Chung, C.P.; Lee, S.J. Enhanced bone formation by transforming growth factor-beta1-releasing collagen/chitosan microgranules. J. Biomed. Mater. Res. A 2006, 76, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Ueda, H.; Yamamoto, M.; Tabata, Y.; Mikos, A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm. Res. 2008, 25, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Ennett, A.B.; Kaigler, D.; Mooney, D.J. Temporally regulated delivery of VEGF in vitro and in vivo. J. Biomed. Mater. Res. A 2006, 79, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur. J. Pharm. Sci. 2015, 66, 173–183. [Google Scholar] [CrossRef]
- Garrett, I.R.; Gutierrez, G.E.; Rossini, G.; Nyman, J.; McCluskey, B.; Flores, A.; Mundy, G.R. Locally delivered lovastatin nanoparticles enhance fracture healing in rats. J. Orthop. Res. 2007, 25, 1351–1357. [Google Scholar] [CrossRef]
- Yoshii, T.; Hafeman, A.E.; Esparza, J.M.; Okawa, A.; Gutierrez, G.; Guelcher, S.A. Local injection of lovastatin in biodegradable polyurethane scaffolds enhances bone regeneration in a critical-sized segmental defect in rat femora. J. Tissue Eng. Regen. Med. 2014, 8, 589–595. [Google Scholar] [CrossRef]
- Tai, I.C.; Fu, Y.C.; Wang, C.K.; Chang, J.K.; Ho, M.L. Local delivery of controlled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int. J. Nanomed. 2013, 8, 3895–3904. [Google Scholar]
- Nyan, M.; Miyahara, T.; Noritake, K.; Hao, J.; Rodriguez, R.; Kuroda, S.; Kasugai, S. Molecular and tissue responses in the healing of rat calvarial defects after local application of simvastatin combined with alpha tricalcium phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 65–73. [Google Scholar] [CrossRef]
- Lourenco, A.H.; Neves, N.; Ribeiro-Machado, C.; Sousa, S.R.; Lamghari, M.; Barrias, C.C.; Trigo Cabral, A.; Barbosa, M.A.; Ribeiro, C.C. Injectable hybrid system for strontium local delivery promotes bone regeneration in a rat critical-sized defect model. Sci. Rep. 2017, 7, 5098. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Tanner, S.; Barker, D.A.; Green, D.; Botchwey, E.A. Delivery of S1P receptor-targeted drugs via biodegradable polymer scaffolds enhances bone regeneration in a critical size cranial defect. J. Biomed. Mater. Res. A 2014, 102, 1210–1218. [Google Scholar] [CrossRef]
- Petrie Aronin, C.S.L.; Tholpady, S.; Tholpady, A.; Sadik, K.; Macdonald, T.; Peirce, S.; Wamhoff, B.; Lynch, K.; Ogle, R.; Botchwey, E. FTY720 promotes loca microvascular network formation and regeneration of cranial bone defects. Tissue Eng. Part A 2010, 16, 1801–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeppner, L.H.; Secreto, F.J.; Westendorf, J.J. Wnt signaling as a therapeutic target for bone diseases. Expert Opin. Targets 2009, 13, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Newman, M.R.; Ackun-Farmmer, M.; Baranello, M.P.; Sheu, T.J.; Puzas, J.E.; Benoit, D.S.W. Fracture-Targeted Delivery of beta-Catenin Agonists via Peptide-Functionalized Nanoparticles Augments Fracture Healing. ACS Nano 2017, 11, 9445–9458. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, M.; Sawamoto, K.; Almeciga-Diaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, G.; Chi, H.; Wang, D.; Tu, C.; Pan, L.; Zhu, L.; Qiu, F.; Guo, F.; Zhu, X. Alendronate-conjugated amphiphilic hyperbranched polymer based on Boltorn H40 and poly(ethylene glycol) for bone-targeted drug delivery. Bioconjug. Chem. 2012, 23, 1915–1924. [Google Scholar] [CrossRef]
- Wang, G.; Mostafa, N.Z.; Incani, V.; Kucharski, C.; Uludag, H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J. Biomed. Mater. Res. A 2012, 100, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Rotman, S.G.; Grijpma, D.W.; Richards, R.G.; Moriarty, T.F.; Eglin, D.; Guillaume, O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J. Control Release 2018, 269, 88–99. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, X.; Huang, J.; Huang, H.; Zou, P.; Hu, J. Atorvastatin-loaded micelles with bone-targeted ligand for the treatment of osteoporosis. Drug Deliv. 2017, 24, 1067–1076. [Google Scholar] [CrossRef] [Green Version]
- Tanigo, T.; Takaoka, R.; Tabata, Y. Sustained release of water-insoluble simvastatin from biodegradable hydrogel augments bone regeneration. J. Control Release 2010, 143, 201–206. [Google Scholar] [CrossRef]
- Low, S.A.; Galliford, C.V.; Jones-Hall, Y.L.; Roy, J.; Yang, J.; Low, P.S.; Kopecek, J. Healing efficacy of fracture-targeted GSK3beta inhibitor-loaded micelles for improved fracture repair. Nanomedicine 2017, 12, 185–193. [Google Scholar] [CrossRef]
- Low, S.A.; Galliford, C.V.; Yang, J.; Low, P.S.; Kopecek, J. Biodistribution of Fracture-Targeted GSK3beta Inhibitor-Loaded Micelles for Improved Fracture Healing. Biomacromolecules 2015, 16, 3145–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.N.; O’Connor, J.P. Osteoclast depletion with clodronate liposomes delays fracture healing in mice. J. Orthop. Res. 2017, 35, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Tabata, Y. Dual-controlled release system of drugs for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control Release 2016, 225, 152–169. [Google Scholar] [CrossRef] [PubMed]
- van der Stok, J.; Wang, H.; Amin Yavari, S.; Siebelt, M.; Sandker, M.; Waarsing, J.H.; Verhaar, J.A.; Jahr, H.; Zadpoor, A.A.; Leeuwenburgh, S.C.; et al. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors. Tissue Eng. Part A 2013, 19, 2605–2614. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ju, W.; Shang, P.; Lei, L.; Nie, H.M. Core-shell microspheres delivering FGF-2 and BMP-2 in different release patterns for bone regeneration. J. Mater. Chem. B 2015, 3, 1907–1920. [Google Scholar] [CrossRef]
- Hernandez, A.; Reyes, R.; Sanchez, E.; Rodriguez-Evora, M.; Delgado, A.; Evora, C. In vivo osteogenic response to different ratios of BMP-2 and VEGF released from a biodegradable porous system. J. Biomed. Mater. Res. A 2012, 100, 2382–2391. [Google Scholar] [CrossRef]
- Geuze, R.E.; Theyse, L.F.; Kempen, D.H.; Hazewinkel, H.A.; Kraak, H.Y.; Oner, F.C.; Dhert, W.J.; Alblas, J. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng. Part A 2012, 18, 2052–2062. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30(14), 2816–2825. [Google Scholar] [CrossRef]
- Sukul, M.; Nguyen, T.B.; Min, Y.K.; Lee, S.Y.; Lee, B.T. Effect of Local Sustainable Release of BMP2-VEGF from Nano-Cellulose Loaded in Sponge Biphasic Calcium Phosphate on Bone Regeneration. Tissue Eng. Part A 2015, 21, 1822–1836. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.; Guldberg, R.; Stayton, P.S. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Dorval Courchesne, N.M.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. USA 2014, 111, 12847–12852. [Google Scholar] [CrossRef] [Green Version]
- Oest, M.E.; Dupont, K.M.; Kong, H.J.; Mooney, D.J.; Guldberg, R.E. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J. Orthop. Res. 2007, 25, 941–950. [Google Scholar] [CrossRef]
- Wildemann, B.; Bamdad, P.; Holmer, C.; Haas, N.P.; Raschke, M.; Schmidmaier, G. Local delivery of growth factors from coated titanium plates increases osteotomy healing in rats. Bone 2004, 34, 862–868. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Ostapowicz, D.; Kandziora, F.; Stange, R.; Haas, N.P.; Raschke, M. Long-term effects of local growth factor (IGF-I and TGF-β1) treatment on fracture healing: A safety study for using growth factors. J. Orthop. Res. 2004, 22, 514–519. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J. Biomed. Mater. Res. 2001, 58, 449–455. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Furuya, H.; Kohara, H.; Tabata, Y. Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials 2011, 32, 2797–2811. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zwingenberger, S.; Langanke, R.; Vater, C.; Lee, G.; Niederlohmann, E.; Sensenschmidt, M.; Jacobi, A.; Bernhardt, R.; Muders, M.; Rammelt, S.; et al. The effect of SDF-1alpha on low dose BMP-2 mediated bone regeneration by release from heparinized mineralized collagen type I matrix scaffolds in a murine critical size bone defect model. J. Biomed. Mater. Res. A 2016, 104, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Schindeler, A.; Gleeson, J.P.; Yu, N.Y.; Cantrill, L.C.; Mikulec, K.; Peacock, L.; O’Brien, F.J.; Little, D.G. A collagen-hydroxyapatite scaffold allows for binding and co-delivery of recombinant bone morphogenetic proteins and bisphosphonates. Acta Biomater. 2014, 10, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Schindeler, A.; Peacock, L.; Mikulec, K.; Baldock, P.A.; Ruys, A.J.; Little, D.G. In vivo local co-delivery of recombinant human bone morphogenetic protein-7 and pamidronate via poly-D, L-lactic acid. Eur. Cell Mater. 2010, 20, 431–442. [Google Scholar] [CrossRef]
- Yu, N.Y.C.; Gdalevitch, M.; Murphy, C.M.; Mikulec, K.; Peacock, L.; Fitzpatrick, J.; Cantrill, L.C.; Ruys, A.J.; Cooper-White, J.J.; Little, D.G.; et al. Spatial Control of Bone Formation Using a Porous Polymer Scaffold Co-Delivering Anabolic Rhbmp-2 and Anti-Resorptive Agents. Eur. Cells Mater. 2014, 27, 98–111. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, E.; Hu, J.; Xue, J.; Zhu, S.; Li, J. Effect of combined local treatment with zoledronic acid and basic fibroblast growth factor on implant fixation in ovariectomized rats. Bone 2009, 44, 225–232. [Google Scholar] [CrossRef]
- Tokuhara, Y.; Wakitani, S.; Imai, Y.; Nomura, C.; Hoshino, M.; Yano, K.; Taguchi, S.; Kim, M.; Kadoya, Y.; Takaoka, K. Local delivery of rolipram, a phosphodiesterase-4-specific inhibitor, augments bone morphogenetic protein-induced bone formation. J. Bone Min. Metab. 2010, 28, 17–24. [Google Scholar] [CrossRef]
- Toyoda, H.; Terai, H.; Sasaoka, R.; Oda, K.; Takaoka, K. Augmentation of bone morphogenetic protein-induced bone mass by local delivery of a prostaglandin E EP4 receptor agonist. Bone 2005, 37, 555–562. [Google Scholar] [CrossRef]
- Kamolratanakul, P.; Hayata, T.; Ezura, Y.; Kawamata, A.; Hayashi, C.; Yamamoto, Y.; Hemmi, H.; Nagao, M.; Hanyu, R.; Notomi, T.; et al. Nanogel-based scaffold delivery of prostaglandin E(2) receptor-specific agonist in combination with a low dose of growth factor heals critical-size bone defects in mice. Arthritis Rheum. 2011, 63, 1021–1033. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Wang, Y.; Yang, G.; Ding, S.; Zhou, S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials 2015, 37, 218–229. [Google Scholar] [CrossRef]
- Moshiri, A.; Sharifi, A.M.; Oryan, A. Role of Simvastatin on fracture healing and osteoporosis: A systematic review on in vivo investigations. Clin. Exp. Pharm. Physiol. 2016, 43, 659–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.S.; Ou, M.E.; Liu, H.; Gu, M.; Lv, L.W.; Fan, C.; Chen, T.; Zhao, X.H.; Jin, C.Y.; Zhang, X.; et al. The effect of simvastatin on chemotactic capability of SDF-1alpha and the promotion of bone regeneration. Biomaterials 2014, 35, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Furuya, H.; Tabata, Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials 2014, 35, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef]
- Madhumathi, K.; Rubaiya, Y.; Doble, M.; Venkateswari, R.; Sampath Kumar, T.S. Antibacterial, anti-inflammatory, and bone-regenerative dual-drug-loaded calcium phosphate nanocarriers-in vitro and in vivo studies. Drug Deliv. Transl. Res. 2018, 8, 1066–1077. [Google Scholar] [CrossRef]
- Decambron, A.; Fournet, A.; Bensidhoum, M.; Manassero, M.; Sailhan, F.; Petite, H.; Logeart-Avramoglou, D.; Viateau, V. Low-dose BMP-2 and MSC dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J. Orthop. Res. 2017, 35, 2637–2645. [Google Scholar] [CrossRef]
- Kirker-Head, C.; Karageorgiou, V.; Hofmann, S.; Fajardo, R.; Betz, O.; Merkle, H.P.; Hilbe, M.; von Rechenberg, B.; McCool, J.; Abrahamsen, L.; et al. BMP-silk composite matrices heal critically sized femoral defects. Bone 2007, 41, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Kanczler, J.M.; Ginty, P.J.; White, L.; Clarke, N.M.; Howdle, S.M.; Shakesheff, K.M.; Oreffo, R.O. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials 2010, 31, 1242–1250. [Google Scholar] [CrossRef]
- Simmons, C.A.; Alsberg, E.; Hsiong, S.; Kim, W.J.; Mooney, D.J. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 2004, 35, 562–569. [Google Scholar] [CrossRef]
- Bretschneider, H.; Quade, M.; Lode, A.; Gelinsky, M.; Rammelt, S.; Zwingenberger, S.; Schaser, K.D.; Vater, C. Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 1412. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S. Mediating bone regeneration by means of drug eluting implants: From passive to smart strategies. Mater Sci Eng C Mater Biol Appl 2017, 71, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Paun, I.A.; Zamfirescu, M.; Luculescu, C.R.; Acasandrei, A.M.; Mustaciosu, C.C.; Mihailescu, M.; Dinescu, M. Electrically responsive microreservoires for controllable delivery of dexamethasone in bone tissue engineering. Appl. Surf. Sci. 2017, 392, 321–331. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface 2011, 8(55), 153–170. [Google Scholar] [CrossRef] [Green Version]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vazquez, E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [Green Version]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.; Ferreira, H.; Reis, R.L.; Neves, N.M. Delivery systems made of natural-origin polymers for tissue engineering and regenerative medicine applications. In Biomaterials from Nature for Advanced Devices and Therapies, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Newman, M.R.; Benoit, D.S. Local and targeted drug delivery for bone regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Patsis, P.A.; Hauser, S.; Voigt, D.; Rothe, R.; Gunther, M.; Cui, M.; Yang, X.; Wieduwild, R.; Eckert, K.; et al. Cytocompatible, Injectable, and Electroconductive Soft Adhesives with Hybrid Covalent/Noncovalent Dynamic Network. Adv. Sci. 2019, 6, 1802077. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.R.; Clark, A.Y.; Garcia, A.J. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J. Biomed. Mater. Res. A 2016, 104, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, I.S.; Cho, T.H.; Kim, H.C.; Yoon, S.J.; Choi, J.; Park, Y.; Sun, K.; Hwang, S.J. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J. Biomed. Mater. Res. A 2010, 95, 673–681. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Weber, F.E.; Schmoekel, H.G.; Schense, J.C.; Kohler, T.; Muller, R.; Hubbell, J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003, 21, 513–518. [Google Scholar] [CrossRef]
- Terella, A.; Mariner, P.; Brown, N.; Anseth, K.; Streubel, S.O. Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Arch. Facial Plast. Surg. 2010, 12, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.W.; Olabisi, R.M.; Olmsted-Davis, E.A.; Davis, A.R.; West, J.L. Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. J. Biomed. Mater. Res. A 2011, 98, 53–62. [Google Scholar] [CrossRef]
- Pan, H.; Kopeckova, P.; Wang, D.; Yang, J.; Miller, S.; Kopecek, J. Water-soluble HPMA copolymer--prostaglandin E1 conjugates containing a cathepsin K sensitive spacer. J. Drug Target 2006, 14, 425–435. [Google Scholar] [CrossRef]
- Pan, H.; Sima, M.; Miller, S.C.; Kopeckova, P.; Yang, J.; Kopecek, J. Efficiency of high molecular weight backbone degradable HPMA copolymer-prostaglandin E1 conjugate in promotion of bone formation in ovariectomized rats. Biomaterials 2013, 34, 6528–6538. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Zhang, Y.; Chen, Y.H.; Dusad, A.; Yuan, H.; Ren, K.; Li, F.; Fehringer, E.V.; Purdue, P.E.; Goldring, S.R.; et al. Simvastatin prodrug micelles target fracture and improve healing. J. Control Release 2015, 200, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jia, Z.; Yuan, H.; Dusad, A.; Ren, K.; Wei, X.; Fehringer, E.V.; Purdue, P.E.; Daluiski, A.; Goldring, S.R.; et al. The Evaluation of Therapeutic Efficacy and Safety Profile of Simvastatin Prodrug Micelles in a Closed Fracture Mouse Model. Pharm. Res. 2016, 33, 1959–1971. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wang, J.; Hou, J.; Guo, H.; Liu, C. Bone regeneration using cell-mediated responsive degradable PEG-based scaffolds incorporating with rhBMP-2. Biomaterials 2013, 34, 1514–1528. [Google Scholar] [CrossRef]
- Gong, T.; Liu, T.; Zhang, L.; Ye, W.; Guo, X.; Wang, L.; Quan, L.; Pan, C. Design Redox-Sensitive Drug-Loaded Nanofibers for Bone Reconstruction. ACS Biomater. Sci. Eng. 2017, 4, 240–247. [Google Scholar] [CrossRef]
- Gan, Q.; Zhu, J.Y.; Yuan, Y.; Liu, H.L.; Qian, J.C.; Lib, Y.S.; Liu, C.S. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J. Mater. Chem. B 2015, 3, 2056–2066. [Google Scholar] [CrossRef]
- Lopez-Noriega, A.; Ruiz-Hernandez, E.; Quinlan, E.; Storm, G.; Hennink, W.E.; O’Brien, F.J. Thermally triggered release of a pro-osteogenic peptide from a functionalized collagen-based scaffold using thermosensitive liposomes. J. Control Release 2014, 187, 158–166. [Google Scholar] [CrossRef]
- Reis, B.; Vehlow, D.; Rust, T.; Kuckling, D.; Müller, M. Thermoresponsive Catechol Based-Polyelectrolyte Complex Coatings for Controlled Release of Bortezomib. Int. J. Mol. Sci. 2019, 20, 6081. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shao, J.; Abd El Raouf, M.; Xie, H.; Huang, H.; Wang, H.; Chu, P.K.; Yu, X.F.; Yang, Y.; AbdEl-Aal, A.M.; et al. Near-infrared light-triggered drug delivery system based on black phosphorus for in vivo bone regeneration. Biomaterials 2018, 179, 164–174. [Google Scholar] [CrossRef]
- Aw, M.S.; Addai-Mensah, J.; Losic, D. A multi-drug delivery system with sequential release using titania nanotube arrays. Chem. Commun. 2012, 48, 3348–3350. [Google Scholar] [CrossRef]
- Aw, M.S.; Addai-Mensah, J.; Losic, D. Magnetic-responsive delivery of drug-carriers using titania nanotube arrays. J. Mater. Chem. 2012, 22, 6561–6563. [Google Scholar] [CrossRef]
- Matsuo, T.; Sugita, T.; Kubo, T.; Yasunaga, Y.; Ochi, M.; Murakami, T. Injectable magnetic liposomes as a novel carrier of recombinant human BMP-2 for bone formation in a rat bone-defect model. J. Biomed. Mater. Res. A 2003, 66, 747–754. [Google Scholar] [CrossRef]
- Crasto, G.J.; Kartner, N.; Reznik, N.; Spatafora, M.V.; Chen, H.; Williams, R.; Burns, P.N.; Clokie, C.; Manolson, M.F.; Peel, S.A. Controlled bone formation using ultrasound-triggered release of BMP-2 from liposomes. J. Control Release 2016, 243, 99–108. [Google Scholar] [CrossRef]
- Jung, F.; Pietzsch, J. Regulation of bone regeneration. Clin. Hemorheol. Microcirc. 2019, 73, 379–380. [Google Scholar] [CrossRef] [Green Version]
- Laube, M.; Kniess, T.; Pietzsch, J. Radiolabeled COX-2 inhibitors for non-invasive visualization of COX-2 expression and activity--a critical update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef] [Green Version]
- Laube, M.; Kniess, T.; Pietzsch, J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy—a hypothesis-driven review. Antioxidants 2016, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Jiang, W.; Zhao, T.; Aifantis, K.E.; Wang, H.; Lin, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Li, X. The application of nanomaterials in controlled drug delivery for bone regeneration. J. Biomed. Mater. Res. A 2015, 103, 3978–3992. [Google Scholar] [CrossRef]










| Adjuvant Drugs | Effect on Bone Metabolism |
|---|---|
| Growth factors | act during all fracture healing stages; stimulate proliferation and differentiation of bone forming cells as well as angiogenesis |
| BMP-2/BMP-7 | |
| FGF-2 | |
| IGF | |
| PDGF | |
| TGF-ß | |
| VEGF | |
| Hormones | acts during all fracture healing stages; anabolic and catabolic effects on bone healing depending on dose and administration |
| Parathyroid hormone | |
| Bisphosphonates | act during several fracture healing stages; prevent bone resorption and increase bone mineralization |
| Nitrogen-containing bisphosphonates | |
| Alendronate | |
| Ibandronate | |
| Pamidronate | |
| Zoledronate | |
| Non-nitrogen-containing bisphosphonates | |
| Clodronate | |
| Glucocorticoids | interferes in late fracture healing phases; inhibits osteoclasto- and osteoblastogenesis; low dose of short-acting glucocorticoids may not be adverse |
| Dexamethasone | |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | elicit anti-inflammatory effects due to inhibition of cyclooxygenases and reduction of prostaglandin production; mainly impair bone repair, especially during the first crucial bone healing phases |
| Ibuprofen | |
| Indomethacin | |
| Prostaglandins | important during early fracture healing phases; biphasic effect on osteoblasts and osteoclasts; intermittent application recommended |
| Prostaglandin E1 | |
| Prostaglandin receptor agonist | |
| Enzyme inhibitors | |
| GSK-3ß inhibitors | GSK-3ß inhibitors prevent proteasomal degradation of β-catenin leading to cytosolic accumulation and nuclear translocation of β-catenin for transcriptional activation of various target genes |
| 603287-31-8 | |
| AZD2858 or | |
| GSK-3ß inhibitor XXVII (AZD2858 × HCl) | |
| Phosphodiesterase-4 inhibitor | phosphodiesterase-4 inhibitor elicits anti-inflammatory effects and increases proliferation and differentiation of osteoblasts and osteoclasts; low doses used for short-term treatment are recommended |
| Rolipram | |
| Proteasome inhibitor | proteasome inhibitor promotes osteoblastogenesis as well as inhibits osteoclastogenesis; low doses used for short-term treatment are recommended |
| Bortezomib | |
| Sphingosine 1-phosphate receptor agonists | increase angiogenesis and osteogenesis |
| FTY720 | |
| SEW2871 | |
| VPC0191 | |
| HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase inhibitors-statins | promote osteogenesis and appear to be anti-inflammatory and pro-angiogenic |
| Lovastatin | |
| Pravastatin | |
| Simvastatin | |
| Atorvastatin | |
| Fluvastatin | |
| Pitavastatin | |
| Rosuvastatin | |
| Divalent metal ions | enhances bone formation and mechanical strength; suppresses bone resorption |
| Strontium | |
| Antibiotics | prevent bone infections; tetracycline inhibits osteoclast differentiation and is high affine to bone minerals |
| Gentamicin | |
| Tetracycline |
| Drug Delivery System | Single (S) or Dual (D) Compound | Drug Release Kinetics | Passive (P) or Triggered (T) Release | Ref. |
|---|---|---|---|---|
| Growth factors | ||||
| calcium phosphate ceramics | S | co-precipitation: 40%, adsorption: 80% (VEGF after 4 days in vitro) | P | [57] |
| PLGA scaffolds | S | unconjugated scaffold: 100% within 4 h, heparin-conjugated scaffold: 100% after 21 days (BMP-2 in vitro) | P | [78] |
| chitosan-silica membranes | S | hybrid membrane: 1.5 µg/mL, chitosan membrane: <0.5 µg/mL (BMP-2 in vitro) | P | [84] |
| gelatin hydrogel | S | reduced water content resulted in a longer BMP-2 retention in vivo | P | [120,121] |
| silk hydrogel | S | 1% silk: 35%, 2% silk: 15% (BMP-2 day 1 in vitro) | P | [122] |
| PLGA-based fibrous scaffold | S | absorption: burst (80% BMP-2 within 1 week), encapsulation: sustained release (80% BMP-2 after 35 days in vitro) | P | [133] |
| nanofibrous membranes | S | 500 pg/day BMP-2 release rate (in vitro) | P | [134] |
| core-shell microspheres | D | core: 80% within 24 days, shell: 80% within 6 days (BMP-2 and FGF-2 in vitro) | P | [170] |
| PLGA microspheres within porous PLGA cylinder | D | 20% (BMP-2) or 10% (VEGF) remaining (14 days in vivo) | P | [171] |
| PLGA microspheres within gelatin hydrogel | D | microspheres: <20% BMP-2 after 30 days, hydrogel: >70% VEGF within 7 days (in vitro) | P | [173] |
| calcium phosphate scaffold loaded with nanocellulose | D | single drug carrier: 3.19% BMP-2 and 7.91% VEGF, dual drug carrier: 3.67% BMP-2 and 4.68% VEGF (day 1 in vitro) | P | [174] |
| PLGA nanoparticles and alginate microcapsules | D | sequential release: 100 ng within 4 days (BMP-2) and 14 days (VEGF) in vitro | P | [178] |
| PLA-coated implants | D | 54% IGF-I and 48% TGF-β1 within 48 h (in vitro) | P | [183] |
| gelatin hydrogels | D | 70–80% SDF-1 and 45–55% BMP-2 (day 1 in vivo) | P | [184] |
| silk microspheres within hydroxyapatite scaffold | D | BMP-2 adsorption: >80% within 7 days, BMP-2 encapsulation: >60% within 14 days (in vitro) | P | [185] |
| heparinized mineralized collagen type I matrix scaffolds | D | 4–10% BMP-2 and ~0.5% SDF-1α of loaded growth factors released after 6 weeks in vitro | P | [186] |
| alginate fibers within PLA polymer | D | sequential release: 2500 pg/mL after 2–3 weeks (BMP-2) and 28 days (VEGF) in vitro | P | [202] |
| PEG-hydrogel | S | VEGF release and scaffold degradation within 2–3 days (50 µg/mL collagenase in vitro) | T | [216] |
| sono-disruptable liposomes | S | 30 s: 5 µg/mL, 60 s: 7 µg/mL (BMP-2, 1 MPa, in vitro) | T | [234] |
| Hormones | ||||
| layered scaffold | S | daily pulsatile PTH release over 21 days (in vitro), 98.5% loading efficiency | P | [76,77] |
| thermo-sensitive liposomes | S | stimulus at day 3: >20%, stimulus at day 8: <10% (PTHrP, 42 °C in vitro) | T | [228] |
| Bisphosphonates | ||||
| collagen sponge | S | 50% ibandronate after 50 h (in vitro) | P | [43] |
| calcium phosphate scaffolds | S | 1 mg/scaffold: 31.33% ± 1.58%, 5 mg/scaffold: 7.99% ± 0.08% (alendronate, within 1 day in vitro), >72% loading efficiency | P | [65] |
| hydroxyapatite-coated titanium implants | S | burst release order: zoledronate > ibandronate > pamidronate (within 7 days in vitro) | P | [104] |
| PLA-calcium phosphate-coated magnesium-based alloys | S | 14% within 3 days: diffusion, up to 27% within 4 weeks: degradation of implant coating (zoledronate, in vitro) | P | [110] |
| hydroxyapatite nanoparticles | S | >60% zoledronate after 1 h (in vitro), loading efficiency between 28.15 ± 4.78% and 52.14 ± 8.47% | P | [148] |
| hydroxyapatite-coated titanium implants | D | dual drug loading reduced initial burst compared to single drug coating by almost 40% at day 1 (zoledronate and bFGF, in vitro) | P | [190] |
| redox-sensitive nanofibers | S | ~20% BMP-2 release by stepwise increase in glutathione concentration (in vitro) | T | [226] |
| Glucocorticoids | ||||
| nanoparticle-embedded electrospun nanofiber | D | BMP-2: 30% after 300 h, dexamethasone: 30% within 100 h (in vitro) | P | [194] |
| polypyrrole-filled electrically responsive microreservoires | S | ~20% dexamethasone release by each stimulus (voltage cycles between −1 V and +1 V in vitro) | T | [208] |
| chitosan-functionalized mesoporous silica nanoparticles | D | pH 6.0: 80% after 50 min, pH 7.4: 10% after 80 min (dexamethasone, in vitro) | T | [227] |
| NSAIDs | ||||
| micelle-loaded titania nanotube arrays | D | sequential release due ratio of micelle (hydrophobic indomethacin) to inverted micelle (hydrophilic gentamicin) (in vitro) | T | [231] |
| Prostaglandin E2 receptor agonist | ||||
| PEG nanogel | D | ~30% released within 30 min, ~70% remained for 7 days (prostaglandin E2 receptor agonist, in vitro) | P | [193] |
| Enzyme inhibitors | ||||
| micelles | S | >50% GSK3β inhibitor in 5 h (in plasma at 37 °C) | P | [165] |
| polyelectrolyte particulate coating | S | ~50% bortezomib release at stimulus (42 °C in vitro) | T | [229] |
| Sphingosine 1-phosphate receptor agonists | ||||
| PLGA-coated allografts | S | 0.57 mg FTY720 released in 14 days in vitro; 64% loading efficiency | P | [92] |
| polymeric microspheres | S | slow-degrading (more hydrophobic): >25%, fast-degrading: <10% (FTY720, after 20 min in vitro) | P | [154] |
| Statins | ||||
| calcium sulfate scaffolds | S | >70% BMP-2 after 14 days in vitro (higher loading reduced release rate) | P | [58] |
| polyurethane scaffolds | S | almost linear trend 200 µg/g foam: 20%, 20 µg/g foam: 10% (lovastatin, after 30 days in vitro) | P | [73] |
| PLGA membranes | S | 1 µg/day release rate (fluvastatin, in vitro) | P | [75] |
| PCL nanofibers | S | absorption: burst, incorporation during fabrication: sustained release (simvastatin) | P | [85] |
| PLGA-PEG hydrogel | S | >50% simvastatin after 2 days (in vitro) | P | [115] |
| PLGA-hydroxyapatite microspheres | S | >20% simvastatin after 1 day (in vitro) | P | [151] |
| PEG-based micelles | S | 60% atorvastatin after 10 h (in vitro) | P | [162] |
| Metal ions | ||||
| PLGA scaffold containing black phosphorus | S | 10 s: 37%, 300 s: 45% (strontium, light irradiation) | T | [230] |
| Antibiotics | ||||
| calcium phosphate carrier | D | calcium-deficient hydroxyapatite: 70% loading (50% tetracycline release after 20 h), hydroxyapatite: 55% loading (50% tetracycline release in 5 h in vitro) | P | [199] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rothe, R.; Hauser, S.; Neuber, C.; Laube, M.; Schulze, S.; Rammelt, S.; Pietzsch, J. Adjuvant Drug-Assisted Bone Healing: Advances and Challenges in Drug Delivery Approaches. Pharmaceutics 2020, 12, 428. https://doi.org/10.3390/pharmaceutics12050428
Rothe R, Hauser S, Neuber C, Laube M, Schulze S, Rammelt S, Pietzsch J. Adjuvant Drug-Assisted Bone Healing: Advances and Challenges in Drug Delivery Approaches. Pharmaceutics. 2020; 12(5):428. https://doi.org/10.3390/pharmaceutics12050428
Chicago/Turabian StyleRothe, Rebecca, Sandra Hauser, Christin Neuber, Markus Laube, Sabine Schulze, Stefan Rammelt, and Jens Pietzsch. 2020. "Adjuvant Drug-Assisted Bone Healing: Advances and Challenges in Drug Delivery Approaches" Pharmaceutics 12, no. 5: 428. https://doi.org/10.3390/pharmaceutics12050428