Quality by Design Optimization of Cold Sonochemical Synthesis of Zidovudine-Lamivudine Nanosuspensions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
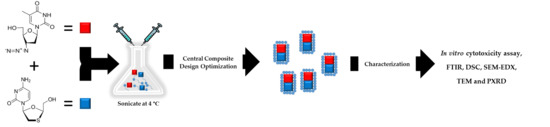
2.2.1. Nano Co-Crystal Synthesis
2.2.2. Nano Co-Crystal Optimization
2.2.3. Particle Size Analysis
2.2.4. Zeta Potential
2.2.5. Formulation Optimization
2.3. Characterization of Optimized Nano Co-Crystal (OPT-NCC)
2.3.1. Differential Scanning Calorimetry
2.3.2. Energy-Dispersive X-ray Spectroscopy Scanning Electron Microscopy
2.3.3. FTIR Spectroscopy
2.3.4. Powder X-ray Diffraction (PXRD)
2.3.5. Transmission Electron Microscopy (TEM)
2.3.6. In Vitro Cytotoxicity Studies
3. Results
3.1. Optimization of Electrosteric NCC
3.1.1. Response Surface Quadratic Model for PS (Y1)
3.1.2. Response Surface Model for PDI (Y2)
3.1.3. Response Surface Model for ZP (Y3)
3.1.4. Formulation Optimization
3.2. Characterization of OPT-NCC
3.2.1. Differential Scanning Calorimetry
3.2.2. SEM-EDX
3.2.3. FTIR Spectroscopy
3.2.4. PXRD
3.2.5. Transmission Electron Microscopy (TEM)
3.2.6. Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV/AIDS Statistics—2019 Fact Sheet; UNAIDS: Geneva, Switzerland, 2019. [Google Scholar]
- Sekhon, B. Pharmaceutical co-crystals—A review. ARS Pharm. 2009, 150, 99–117. [Google Scholar]
- Brittain, H.G. Cocrystal Systems of Pharmaceutical Interest: 2010. Cryst. Growth Des. 2011, 36, 361–381. [Google Scholar] [CrossRef]
- Gao, Y.; Zu, H.; Zhang, J. Enhanced dissolution and stability of adefovir dipivoxil by cocrystal formation. J. Pharm. Pharmacol. 2011, 63, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bethune, S.J.; Schultheiss, N.; Henck, J.O. Improving the poor aqueous solubility of nutraceutical compound pterostilbene through cocrystal formation. Cryst. Growth Des. 2011, 11, 2817–2823. [Google Scholar] [CrossRef]
- Jayasankar, A.; Reddy, L.S.; Bethune, S.J.; Rodríguez-Hornedo, N. Role of Cocrystal and Solution Chemistry on the Formation and Stability of Cocrystals with Different Stoichiometry. Cryst. Growth Des. 2009, 9, 889–897. [Google Scholar] [CrossRef]
- Sekhon, B.S. Drug-drug co-crystals. DARU J. Pharm. Sci. 2012, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. Antiretroviral drugs. Curr. Opin. Pharmacol. 2010, 10, 507–515. [Google Scholar] [CrossRef]
- Breckenridge, A. Pharmacology of drugs for HIV. Medicine 2009, 37, 374–377. [Google Scholar] [CrossRef]
- Tsibris, A.M.N.; Hirsch, M.S. Antiretroviral Therapy for Human Immunodeficiency Virus Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Eighth Edition), M.J.B.T.-M., Eds.; Elsevier: Philadelphia, PA, USA, 2014; Volume 1, pp. 1622–1641.e6. ISBN 9996096742. [Google Scholar]
- Wainberg, M.A. AIDS: Drugs that prevent HIV infection. Nature 2011, 469, 306–307. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Azim, Y.; Thakur, T.S.; Desiraju, G.R. Co-Crystals of the Anti-HIV Drugs Lamivudine and Zidovudine. Cryst. Growth Des. 2009, 9, 951–957. [Google Scholar] [CrossRef]
- Xia, D.; Gan, Y.; Cui, F. Application of Precipitation Methods for the Production of Water-insoluble Drug Nanocrystals: Production Techniques and Stability of Nanocrystals. Curr. Pharm. Des. 2014, 20, 408–435. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, S.V.; Yadav, M.D. Effect of ultrasound and stabilizers on nucleation kinetics of curcumin during liquid antisolvent precipitation. Ultrason. Sonochem. 2015, 24, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Pi, J.; Liu, Z.; Wang, H.; Gu, X.; Wang, S.; Zhang, B.; Luan, H.; Zhu, Z. Ursolic Acid Nanocrystals for Dissolution Rate and Bioavailability Enhancement: Influence of Different Particle Size. Curr. Drug Deliv. 2016, 13, 1358–1366. [Google Scholar] [CrossRef]
- Nakarani, M.; Patel, P.; Patel, J.; Patel, P.; Murthy, R.S.R.; Vaghani, S.S. Cyclosporine A-Nanosuspension: Formulation, characterization and in vivo comparison with a marketed formulation. Sci. Pharm. 2010, 78, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Pharmaceutical Nanotechnology Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef]
- Iurian, S.; Bogdan, C.; Tomuță, I.; Szabó-Révész, P.; Chvatal, A.; Leucuța, S.E.; Moldovan, M.; Ambrus, R. Development of oral lyophilisates containing meloxicam nanocrystals using QbD approach. Eur. J. Pharm. Sci. 2017, 104, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.F.; Wan, K.Y.; Cheng, K.K.; Wong, K.W.; Sun, C.C.; Baum, L.; Chow, A.H.L. Development of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilization. Eur. J. Pharm. Biopharm. 2015, 94, 436–449. [Google Scholar] [CrossRef]
- Dong, Y.; Ng, W.K.; Shen, S.; Kim, S.; Tan, R.B.H. Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation. Int. J. Pharm. 2009, 375, 84–88. [Google Scholar] [CrossRef]
- Leng, D.; Chen, H.; Li, G.; Guo, M.; Zhu, Z.; Xu, L.; Wang, Y. Development and comparison of intramuscularly long-acting paliperidone palmitate nanosuspensions with different particle size. Int. J. Pharm. 2014, 472, 380–385. [Google Scholar] [CrossRef]
- De Smet, L.; Saerens, L.; De Beer, T.; Carleer, R.; Adriaensens, P.; Van Bocxlaer, J.; Vervaet, C.; Remon, J.P. Formulation of itraconazole nanococrystals and evaluation of their bioavailability in dogs. Eur. J. Pharm. Biopharm. 2014, 87, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Witika, B.A.; Smith, V.J.; Walker, R.B. A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self- assembling Nano Co-crystals. Pharmaceutics 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowacek, A.S.; Mcmillan, J.; Miller, R.; Anderson, A.; Rabinow, B.; Gendelman, H.E. Macrophages: Implications for NeuroAIDS Therapeutics. J. Neuroimmune Pharmacol. 2012, 5, 592–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orenstein, J.M. Replication of HIV-1 in vivo and in vitro. Ultrastruct. Pathol. 2007, 31, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Koppensteiner, H.; Wu, L. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Van’t Klooster, G.; Hoeben, E.; Borghys, H.; Looszova, A.; Bouche, M.-P.P.; Van Velsen, F.; Baert, L. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob. Agents Chemother. 2010, 54, 2042–2050. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Jefferies, C.; Cryan, S.-A. Targeted Liposomal Drug Delivery to Monocytes and Macrophages. J. Drug Deliv. 2011, 2011, 727241. [Google Scholar] [CrossRef]
- Bender, A.; Schäfer, V.; Steffan, A.M.; Royer, C.; Kreuter, J.; Rübsamen-Waigmann, H.; von Briesen, H. Inhibition of HIV in vitro by antiviral drug-targeting using nanoparticles. Res. Virol. 1994, 145, 215–220. [Google Scholar] [CrossRef]
- Désormeaux, A.; Bergeron, M.G. Lymphoid tissue targeting of anti-HIV drugs using liposomes. Methods Enzymol. 2005, 391, 330–351. [Google Scholar]
- Bučar, D.-K.; MacGillivray, L.R. Preparation and Reactivity of Nanocrystalline Cocrystals Formed via Sonocrystallization. J. Am. Chem. Soc. 2007, 129, 32–33. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, L.R.; Sander, J.R.; Bucar, D.K.; Elacqua, E.; Zhang, G.; Henry, R. Sonochemical synthesis of nano-cocrystals. Proc. Mtgs. Acoust. 2013, 19, 45090. [Google Scholar]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Wu, W. Injected nanocrystals for targeted drug delivery. Acta Pharm. Sin. B 2016, 6, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathisaran, I.; Dalvi, S.V. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Brittain, H.G. Pharmaceutical cocrystals: The coming wave of new drug substances. J. Pharm. Sci. 2013, 102, 311–317. [Google Scholar] [CrossRef]
- Desai, P.P.; Patravale, V.B. Curcumin Cocrystal Micelles—Multifunctional Nanocomposites for Management of Neurodegenerative Ailments. J. Pharm. Sci. 2018, 107, 1143–1156. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Chattopadhyay, N.; Wu, X.Y.; Bendayan, R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv. Drug Deliv. Rev. 2010, 62, 503–517. [Google Scholar] [CrossRef]
- Hill, W.J.; Hunter, W.G. A Review of Response Surface Methodology: A Literature Review. Technometrics 2012, 8, 571–590. [Google Scholar] [CrossRef]
- Matteucci, M.E.; Hotze, M.A.; Johnston, K.P.; Williams, R.O. Drug nanoparticles by antisolvent precipitation: Mixing energy versus surfactant stabilization. Langmuir 2006, 22, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.; Shi, K.; Piao, H.; Piao, H.; Liang, N.; Xia, D.; Cui, F. A novel surface modified nitrendipine nanocrystals with enhancement of bioavailability and stability. Int. J. Pharm. 2012, 430, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Millqvist-Fureby, A.; Elema, M.R.; Hansen, T.; Müllertz, A.; Hovgaard, L. Adsorption of pharmaceutical excipients onto microcrystals of siramesine hydrochloride: Effects on physicochemical properties. Eur. J. Pharm. Biopharm. 2009, 71, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M. Cyclosporine Nanosuspensions: Optimised Size Characterisation and Oral Formulations. Ph.D. Thesis, Freie Universitat, Berlin, Germany, 2006. [Google Scholar]
- Hou, Y.; Shao, J.; Fu, Q.; Li, J.; Sun, J.; He, Z. Spray-dried nanocrystals for a highly hydrophobic drug: Increased drug loading, enhanced redispersity, and improved oral bioavailability. Int. J. Pharm. 2017, 516, 372–379. [Google Scholar] [CrossRef]
- Rachmawati, H.; Rahma, A.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Destabilization mechanism of ionic surfactant on curcumin nanocrystal against electrolytes. Sci. Pharm. 2016, 84, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Dzakwan, M.; Pramukantoro, G.E.; Mauludin, R.; Wikarsa, S. Formulation and characterization of fisetin nanosuspension. In IOP Conference Series: Materials Science and Engineering; IOP: London, UK, 2017; Volume 259. [Google Scholar]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surfaces B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef]
- Lopeandía, A.F.; Rodríguez-Viejo, J. Size-dependent melting and supercooling of Ge nanoparticles embedded in a SiO2 thin film. Thermochim. Acta 2007, 461, 82–87. [Google Scholar] [CrossRef]
- Sun, J.; Simon, S.L. The melting behavior of aluminum nanoparticles. Thermochim. Acta 2007, 463, 32–40. [Google Scholar] [CrossRef]
- Palermo, E.F.; Chiu, J. Critical review of methods for the determination of purity by differential scanning calorimetry. Thermochim. Acta 1976, 14, 1–12. [Google Scholar] [CrossRef]
- Brink, G. Infrared Studies of Water in Crystalline Hydrates: Ba(ClO3)2·H2O. Appl. Spectrosc. 1976, 30, 630–631. [Google Scholar] [CrossRef]
- Falk, M.; Huang, C.-H.; Knop, O. Infrared Spectra of Water in Crystalline Hydrates: KSnCl3.H2O, an Untypical Monohydrate. Can. J. Chem. 2006, 52, 2928–2931. [Google Scholar] [CrossRef] [Green Version]
- Kamb, W.B. Theory of Preferred Crystal Orientation Developed. J. Geol. 1958, 67, 153–170. [Google Scholar] [CrossRef]
- Lang, A.R. X-ray diffraction procedures for polycrystal-line and amorphous materials. Acta Metall. 1956, 4, 102. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Q.; Gu, N. Preparation of all-trans retinoic acid nanosuspensions using a modified precipitation method. Drug Dev. Ind. Pharm. 2006, 32, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Romberg, B.; Hennink, W.E.; Storm, G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008, 25, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, P.; Bothiraja, C.; Pawar, A. Methotrexate-Loaded Nanomixed Micelles: Formulation, Characterization, Bioavailability, Safety, and In Vitro Anticancer Study. J. Pharm. Innov. 2018, 13, 213–225. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake characteristics of liposomes by rat alveolar macrophages: Influence of particle size and surface mannose modification. J. Pharm. Pharmacol. 2007, 59, 75–80. [Google Scholar] [CrossRef]
- Chono, S.; Tauchi, Y.; Morimoto, K. Influence of particle size on the distributions of liposomes to atherosclerotic lesions in mice. Drug Dev. Ind. Pharm. 2006, 32, 125–135. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. J. Drug Target. 2006, 14, 557–566. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C. Buparvaquone mucoadhesive nanosuspension: Preparation, optimisation and long-term stability. Int. J. Pharm. 2002, 237, 151–161. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Khatun, S.; Luna, I.P. Increasing possibilities of nanosuspension. J. Nanotechnol. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suárez, A.I. Targeting to macrophages: Role of physicochemical properties of particulate carriers—Liposomes and microspheres—On the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Lupin Limited. WO 2009/116055 Al 2009; Lupin Limited: Mumbaid, India, 2009. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]









| Std. Run | Form. Code | SDS % w/v | TPGS 1000 % w/v |
|---|---|---|---|
| 4 | 1 | 1.00 | 2.00 |
| 11 | 2 | 0.50 | 1.50 |
| 5 | 3 | 0.00 | 1.50 |
| 2 | 4 | 1.00 | 1.00 |
| 7 | 5 | 0.50 | 0.79 |
| 8 | 6 | 0.50 | 2.21 |
| 13 | 7 | 0.50 | 1.50 |
| 6 | 8 | 1.21 | 1.50 |
| 1 | 9 | 0.00 | 1.00 |
| 3 | 10 | 0.00 | 2.00 |
| 9 | 11 | 0.50 | 1.50 |
| 10 | 12 | 0.50 | 1.50 |
| 12 | 13 | 0.50 | 1.50 |
| Formulation Variable | Optimized Condition |
|---|---|
| SDS concentration (X1) | 0.90% w/v |
| TPGS 1000 concentration (X2) | 1.40% w/v |
| Summary of CCD Experiments for Optimisation | Responses for NCC Produced Using CCD | |||||
|---|---|---|---|---|---|---|
| Std. Run | Form. Code | SDS % w/v | TPGS % w/v | PS nm | PDI | ZP mV |
| 4 | 1 | 1.00 | 2.00 | 451.9 ± 43.1 | 0.689 ± 0.003 | −45.0 ± 3.9 |
| 11 | 2 | 0.50 | 1.50 | 446.8 ± 52.8 | 0.478 ± 0.034 | −27.7 ± 1.2 |
| 5 | 3 | 0.00 | 1.50 | 705.6 ± 92.3 | 0.474 ± 0.092 | −22.3 ± 0.9 |
| 2 | 4 | 1.00 | 1.00 | 475.9 ± 22.3 | 0.506 ± 0.033 | −30.5 ± 2.6 |
| 7 | 5 | 0.50 | 0.79 | 630.1 ± 73.2 | 0.414 ± 0.045 | −27.1 ± 1.4 |
| 8 | 6 | 0.50 | 2.21 | 304.1 ± 38.6 | 0.498 ± 0.087 | −21.9 ± 2.3 |
| 13 | 7 | 0.50 | 1.50 | 436.4 ± 67.1 | 0.471 ± 0.011 | −25.9 ± 3.1 |
| 6 | 8 | 1.21 | 1.50 | 404.9 ± 73.6 | 0.462 ± 0.038 | −37.0 ± 0.3 |
| 1 | 9 | 0.00 | 1.00 | 859.4 ± 101.2 | 0.448 ± 0.088 | −13.3 ± 3.7 |
| 3 | 10 | 0.00 | 2.00 | 656.9 ± 77.2 | 0.284 ± 0.018 | −13.5 ± 2.2 |
| 9 | 11 | 0.50 | 1.50 | 424.9 ± 21.7 | 0.432 ± 0.028 | −26.2 ± 1.6 |
| 10 | 12 | 0.50 | 1.50 | 423.7 ± 13.2 | 0.449 ± 0.049 | −25.3 ± 0.7 |
| 12 | 13 | 0.50 | 1.50 | 430.7 ± 52.1 | 0.466 ± 0.009 | −24.8 ± 2.7 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob. > F |
|---|---|---|---|---|---|
| Model | 2.71 × 105 | 5 | 54,111.2 | 26.82 | 0.0002 |
| A-SDS Conc. | 1.68 × 105 | 1 | 1.679 × 105 | 83.23 | <0.0001 |
| B-TGPS Conc. | 59,087.81 | 1 | 59,087.8 | 29.29 | 0.0010 |
| AB | 7965.56 | 1 | 7965.56 | 3.95 | 0.0873 |
| A2 | 73,941.57 | 1 | 73,941.6 | 36.65 | 0.0005 |
| B2 | 6594.86 | 1 | 6594.86 | 3.27 | 0.1135 |
| Residual | 14,121.22 | 7 | 2017.32 | ||
| Lack of Fit | 13,763.08 | 3 | 4587.69 | 51.24 | 0.0012 |
| Pure Error | 358.14 | 4 | 89.54 | ||
| Corr. Total | 2.85 × 105 | 12 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob. > F |
|---|---|---|---|---|---|
| Model | 0.061 | 3 | 0.02 | 6.37 | 0.0132 |
| A-SDS Concentration | 0.029 | 1 | 0.029 | 8.99 | 0.015 |
| B-TGPS Concentration | 2.37 × 10−3 | 1 | 2.37 × 10−3 | 0.74 | 0.412 |
| AB | 0.03 | 1 | 0.03 | 9.39 | 0.0135 |
| Residual | 0.029 | 9 | 3.21 × 10−3 | ||
| Lack of Fit | 0.027 | 5 | 5.496 × 10−3 | 15.9 | 0.0096 |
| Pure Error | 1.38 × 10−3 | 4 | 3.457 × 10−3 | ||
| Corr. Total | 0.09 | 12 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob. > F |
|---|---|---|---|---|---|
| Model | 666.17 | 2 | 333.08 | 17.56 | 0.0005 |
| A-SDS Concentration | 659.42 | 1 | 659.42 | 34.76 | 0.0002 |
| B-TGPS Concentration | 6.75 | 1 | 6.75 | 0.36 | 0.5642 |
| Residual | 189.72 | 10 | 18.97 | ||
| Lack of Fit | 184.85 | 6 | 30.81 | 25.32 | 0.0038 |
| Pure Error | 4.87 | 4 | 1.22 | ||
| Corr. Total | 855.89 | 12 |
| Formulation Variables | Formulation Responses | Desirability | |||
|---|---|---|---|---|---|
| SDS % w/v | TPGS % w/v | PS Nm | PDI | ZP mV | |
| 0.90 | 1.40 | 393.08 | 0.499 | −33.47 | 1.000 |
| Response | Predicted Value | Experimental Value | % Predicted Error |
|---|---|---|---|
| PS nm | 393.08 | 332.9 ± 42.85 | 18.08 |
| PDI | 0.499 | 0.474 ± 0.040 | 5.27 |
| ZP mV | −33.47 | −34.6 ± 5.56 | 3.27 |
| Element | OPT-NCC | Uncoated NCC |
|---|---|---|
| Atomic % | Atomic % | |
| Ck | 56.97 ± 2.18 | 49.67 ± 1.21 |
| Nk | 13.99 ± 0.94 | 20.81 ± 0.94 |
| Ok | 25.87 ± 1.22 | 28.53 ± 1.02 |
| Nak | 0.26 ± 0.13 | - |
| Sk | 2.97 ± 0.34 | 1.00 ± 0.03 |
| Compound | Cell Viability % | SD |
|---|---|---|
| OPT-NCC | 76.9 | 5.0 |
| Physical Mixture | 52.6 | 5.1 |
| AZT | 49.6 | 6.2 |
| 3TC | 62.3 | 4.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witika, B.A.; Smith, V.J.; Walker, R.B. Quality by Design Optimization of Cold Sonochemical Synthesis of Zidovudine-Lamivudine Nanosuspensions. Pharmaceutics 2020, 12, 367. https://doi.org/10.3390/pharmaceutics12040367
Witika BA, Smith VJ, Walker RB. Quality by Design Optimization of Cold Sonochemical Synthesis of Zidovudine-Lamivudine Nanosuspensions. Pharmaceutics. 2020; 12(4):367. https://doi.org/10.3390/pharmaceutics12040367
Chicago/Turabian StyleWitika, Bwalya A., Vincent J. Smith, and Roderick B. Walker. 2020. "Quality by Design Optimization of Cold Sonochemical Synthesis of Zidovudine-Lamivudine Nanosuspensions" Pharmaceutics 12, no. 4: 367. https://doi.org/10.3390/pharmaceutics12040367