Effect of Gold Nanoparticle on 5-Fluorouracil-Induced Experimental Oral Mucositis in Hamsters
Abstract
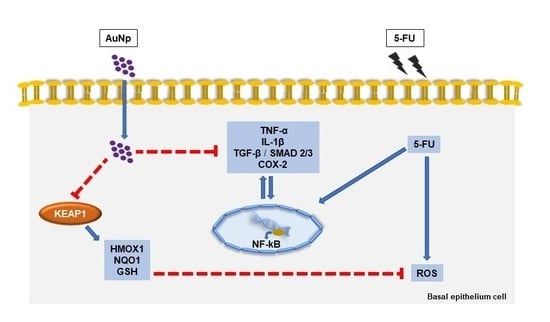
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Gold Nanoparticles (AuNps) Stabilized by Polivynylpyrrolidone (PVP)
2.2. Experimental Induction of 5-Fluorouracil (5-FU) Oral Mucositis in Golden Sirian Hamster Groups
2.3. Macroscopic and Histopathological Analyses
2.4. Assays for Quantification of Cytokines and Reduced Glutathione (GSH)
2.5. Histological Analysis by Immunohistochemistry
2.6. Western Blot for TGF-β and SMAD 2/3
2.7. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
2.8. Digestion of Hepatic and Pulmonary Tissue by Microwave and Quantification of Gold Nanoparticles by Inductive-Coupled Plasma Optical Emission Spectrometry (ICP-OES)
3. Results
3.1. Macroscopic and Histopathological Analysis of Oral Mucosa
3.2. Histopathological Analysis of Liver and Lung and Quantification of the Gold Nanoparticle in Tissues
3.3. Quantification of Cytokines and Glutathione (GSH)
3.4. Immunohistochemistry for COX-2 and NF-κB
3.5. Western Blot for TGF-β and SMAD 2/3
3.6. Quantification of Gene Expression by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3.7. Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Vanhoecke, B.; De Ryck, T.; Stringer, A.; Van de Wiele, T.; Keefe, D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 2015, 21, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Sonis, S.T. Mucositispathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.A.C.X.; Leitão, R.F.C.; MacEdo, R.N.; Barboza, D.R.M.M.; Gomes, A.S.; Nogueira, N.A.P.; Alencar, N.M.N.; Ribeiro, R.A.; Brito, G.A.C. Effect of atorvastatin on 5-fluorouracil-induced experimental oral mucositis. Cancer Chemother. Pharmacol. 2011, 67, 1085–1100. [Google Scholar] [CrossRef]
- Barbosa, M.M.; Araújo, A.A.d.; Júnior, R.F.d.A.; Guerra, G.C.B.; Brito, G.A.d.C.; Leitão, R.C.; Ribeiro, S.B.; Tavares, E.d.A.; Vasconcelos, R.C.; Garcia, V.B.; et al. Telmisartan Modulates the Oral Mucositis Induced by 5-Fluorouracil in Hamsters. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Araújo, A.A.d.; Araújo, L.d.S.; Medeiros, C.A.C.X.d.; Leitão, R.F.d.C.; Brito, G.A.d.C.; Costa, D.V.d.S.; Guerra, G.C.B.; Garcia, V.B.; Lima, M.L.d.S.; Junior, R.F.d.A. Protective effect of angiotensin II receptor blocker against oxidative stress and inflammation in an oral mucositis experimental model. J. Oral Pathol. Med. 2018, 47, 972–984. [Google Scholar] [CrossRef]
- Carvalho, T.G.d.; Garcia, V.B.; Araújo, A.A.d.; Gasparotto, L.H.d.S.; Silva, H.; Guerra, G.C.B.; Miguel, E.d.C.; Leitão, R.F.d.C.; Costa, D.V.d.S.; Cruz, L.J. Spherical neutral Gold nanoparticles improve anti-inflammatory response, oxidative stress and fibrosis in alcohol-methamphetamine-induced liver injury in rats. Int. J. Pharm. 2018, 548, 1–14. [Google Scholar] [CrossRef]
- Ahmad, N.; Bhatnagar, S.; Saxena, R.; Iqbal, D.; Ghosh, A.K.; Dutta, R. Biosynthesis and characterization of gold nanoparticles: Kinetics, in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 553–564. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [Green Version]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J. Pharmacol. Exp. Ther. 2005, 315, 196–202. [Google Scholar] [CrossRef]
- Lamprecht, A. IBD: Selective nanoparticle adhesion can enhance colitis therapy. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Neves, J.d.; Sarmento, B. Nanoparticles for the regulation of intestinal Inflammation opportunites and challenges. Nanomedicine 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Dai, Z.H.; Liu, P.C.; Chuu, J.J.; Lee, K.Y.; Lee, S.L.; Chen, Y.J. Effects of nanogold on the alleviation of carbon tetrachloride-induced hepatic injury in rats. Chin. J. Physiol. 2012. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; De Bem Silveira, G.; De Souza, D.L.; Brandolfi, J.A.; De Souza, C.T.; Paula, M.M.S.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Kandimalla, R.; Sharma, K.K.; Kataki, A.C.; Deka, M.; Kotoky, J. Amoxicillin functionalized gold nanoparticles reverts MRSA resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 720–727. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their comparative effects in experimental inflammation. J. Photochem. Photobiol. B 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Fratoddi, I.; Benassi, L.; Botti, E.; Vaschieri, C.; Venditti, I.; Bessar, H.; Samir, M.A.; Azzoni, P.; Magnoni, C.; Costanzo, A.; et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine 2019, 17, 276–286. [Google Scholar] [CrossRef]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3β signaling pathway. Nanomedicine 2017, 13, 2533–2544. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, L.H.; Garcia, A.C.; Gomes, J.F.; Tremiliosi-Filho, G. Electrocatalytic performance of environmentally friendly synthesized gold nanoparticles towards the borohydride electro-oxidation reaction. J. Power Sources 2012, 218, 73–78. [Google Scholar] [CrossRef]
- De Araujo, R.F.J.; De Araujo, A.A.; Pessoa, J.B.; Freire Neto, F.P.; Da Silva, G.R.; Leitao Oliveira, A.L.; De Carvalho, T.G.; Silva, H.F.; Eugenio, M.; Sant’Anna, C.; et al. Anti-inflammatory, analgesic and anti-tumor properties of gold nanoparticles. Pharm. Rep. 2017, 69, 119–129. [Google Scholar] [CrossRef]
- Sonis, S.T.; Tracey, C.; Shklar, G.; Jenson, J.; Florine, D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 437–443. [Google Scholar] [CrossRef]
- Leitão, R.F.C.; Ribeiro, R.A.; Bellaguarda, E.A.L.; MacEdo, F.D.B.; Silva, L.R.; Oriá, R.B.; Vale, M.L.; Cunha, F.Q.; Brito, G.A.C. Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. Cancer Chemother. Pharmacol. 2007, 59, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S. A Biological Approach to Mucositis. J. Support. Oncol. 2004, 2, 21–36. [Google Scholar] [PubMed]
- Ribeiro, S.B.; De Araújo, A.A.; De Araújo Júnior, R.F.; De Castro Brito, G.A.; Leitão, R.C.; Barbosa, M.M.; Garcia, V.B.; Medeiros, A.C.; De Medeiros, C.A.C.X. Protective effect of dexamethasone on 5-FUinduced oral mucositis in hamsters. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Sonis, S.T.; Peterson, R.L.; Edwards, L.J.; Lucey, C.A.; Wang, L.; Mason, L.; Login, G.; Ymamkawa, M.; Moses, G.; Bouchard, P.; et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000, 36, 373–381. [Google Scholar] [CrossRef]
- De Araujo, A.A.; Varela, H.; De Medeiros, C.A.C.X.; De Castro Brito, G.A.; De Lima, K.C.; De Moura, L.M.; De Araujo, R.F. Azilsartan reduced TNF-α and IL-1β levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-α in an oral mucositis model. PLoS ONE 2015, 10, e0116799. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Kendall, C.; Ionescu-Matiu, I.; Dreesman, G.R. Utilization of the biotin/avidin system to amplify the sensitivity of the enzyme-linked immunosorbent assay (ELISA). J. Immunol. Methods 1983. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Curra, M.; Martins, M.A.T.; Lauxen, I.S.; Pellicioli, A.C.A.; Sant’Ana Filho, M.; Pavesi, V.C.S.; Carrard, V.C.; Martins, M.D. Effect of topical chamomile on immunohistochemical levels of IL-1β and TNF-α in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother. Pharmacol. 2013, 71, 293–299. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.; Lima-Junior, R.C.P.; Martins, C.S.; Leitao, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci. Rep. 2019, 9, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. J. Vis. Exp. Jove 2010, 22. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- Morais, T.; Soares, M.E.; Duarte, J.A.; Soares, L.; Maia, S.; Gomes, P.; Pereira, E.; Fraga, S.; Carmo, H.; Bastos Mde, L. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur. J. Pharm. Biopharm. 2012, 80, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elzey, S.; Tsai, D.-H.; Rabb, S.A.; Lee, L.Y.; Winchester, M.R.; Hackley, V.A. Quantification of ligand packing density on gold nanoparticles using ICP-OES. Anal. Bioanal. Chem. 2012, 403, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O. Selective determination of gold(III) ion using CuO microsheets as a solid phase adsorbent prior by ICP-OES measurement. Talanta 2013, 104, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Amorello, D.; Barreca, S.; Bruno, M.; Milia, A.; Orecchio, S.; Pettignano, A. Chemical characterization of ancient liturgical vestment (chasuble) by Inductively Coupled Plasma–Optical Emission Spectrometry (ICP–OES). Microchem. J. 2016, 129, 305–309. [Google Scholar] [CrossRef]
- Sajnog, A.; Hanć, A.; Barałkiewicz, D. Metrological approach to quantitative analysis of clinical samples by LA-ICP-MS: A critical review of recent studies. Talanta 2018, 182, 92–110. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Abdelhalim, M.A.; Jarrar, B.M. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Hu, Z.; Ma, J.; Wang, X.; Zhang, Y.; Wang, W.; Yuan, Z. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf. B Biointerfaces 2018, 167, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Kingston, M.; Pfau, J.C.; Gilmer, J.; Brey, R. Selective inhibitory effects of 50-nm gold nanoparticles on mouse macrophage and spleen cells. J. Immunotoxicol. 2016, 13, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnson, K.W.; Arany, P.R.; Philip, A. Transforming Growth Factor Beta Signaling in Cutaneous Wound Healing: Lessons Learned from Animal Studies. Adv. Wound Care (New Rochelle) 2013, 2, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedes, R.P.; Dal Bosco, L.; Araujo, A.S.; Bello-Klein, A.; Ribeiro, M.F.; Partata, W.A. Sciatic nerve transection increases gluthatione antioxidant system activity and neuronal nitric oxide synthase expression in the spinal cord. Brain Res. Bull. 2009, 80, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.H.; Shieh, J.M.; Tsou, C.J.; Wu, W.B. Gold nanoparticles induce heme oxygenase-1 expression through Nrf2 activation and Bach1 export in human vascular endothelial cells. Int. J. Nanomed. 2015, 10, 5925–5939. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, X.; Lian, C.; Wu, J.; Fang, Y.; Ye, X. KEAP1/NRF2 axis regulates H2O2-induced apoptosis of pancreatic β-cells. Gene 2019, 691, 8–17. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Skeff, M.A.; Brito, G.A.; De Oliveira, M.G.; Braga, C.M.; Cavalcante, M.M.; Baldim, V.; Holanda-Afonso, R.C.; Silva-Boghossian, C.M.; Colombo, A.P.; Ribeiro, R.A.; et al. S-nitrosoglutathione accelerates recovery from 5-fluorouracil-induced oral mucositis. PLoS ONE 2014, 9, e113378. [Google Scholar] [CrossRef]







| Parameter | Value |
|---|---|
| RF power | 1150 W |
| Nebulizer gas rate | 0.75 L/min |
| Auxiliary gas rate | 0.5 L/min |
| Stabilization time | 30 s |
| Display Mode | Axial |
| Replicates | 3 |
| Wavelength | Au (267.595 nm) |
| Element | LOD (mg/L) | LOQ (mg/L) | Linearity | * Same Day | * Different Days |
|---|---|---|---|---|---|
| Au | 0.0017 | 0.0051 | 0.9997440 | 1.0 | 4.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilar, C.J.F.; Ribeiro, S.B.; de Araújo, A.A.; Guerra, G.C.B.; de Araújo Júnior, R.F.; Brito, G.A.d.C.; Leitão, R.F.C.; Pontes, D.d.L.; Gasparotto, L.H.D.S.; Oliveira, M.M.B.; et al. Effect of Gold Nanoparticle on 5-Fluorouracil-Induced Experimental Oral Mucositis in Hamsters. Pharmaceutics 2020, 12, 304. https://doi.org/10.3390/pharmaceutics12040304
Vilar CJF, Ribeiro SB, de Araújo AA, Guerra GCB, de Araújo Júnior RF, Brito GAdC, Leitão RFC, Pontes DdL, Gasparotto LHDS, Oliveira MMB, et al. Effect of Gold Nanoparticle on 5-Fluorouracil-Induced Experimental Oral Mucositis in Hamsters. Pharmaceutics. 2020; 12(4):304. https://doi.org/10.3390/pharmaceutics12040304
Chicago/Turabian StyleVilar, Carmem Jane Ferreira, Susana Barbosa Ribeiro, Aurigena Antunes de Araújo, Gerlane Coelho Bernardo Guerra, Raimundo Fernandes de Araújo Júnior, Gerly Anne de Castro Brito, Renata Ferreira Carvalho Leitão, Daniel de Lima Pontes, Luiz Henrique Da Silva Gasparotto, Maisie Mitchele Barbosa Oliveira, and et al. 2020. "Effect of Gold Nanoparticle on 5-Fluorouracil-Induced Experimental Oral Mucositis in Hamsters" Pharmaceutics 12, no. 4: 304. https://doi.org/10.3390/pharmaceutics12040304