Novel Carboxylated Chitosan-Based Triptolide Conjugate for the Treatment of Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Synthesis of Triptolide Analog (TPS) [17]
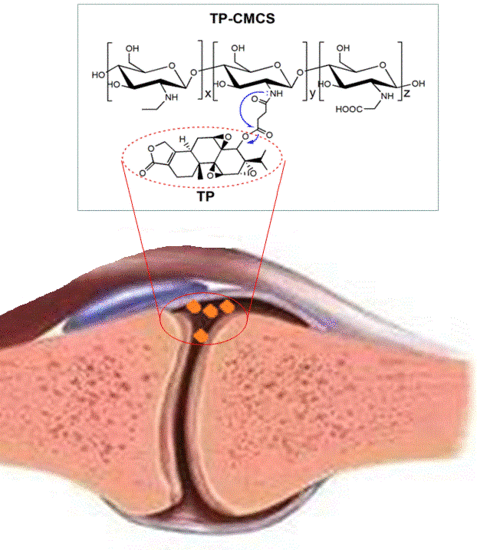
2.3. Preparation of TP-CMCS Conjugates
2.4. The Weight Percentage of Triptolide in the Conjugate
2.5. Properties Studies
2.6. In Vitro Cytotoxicity Study
2.7. Analysis of Apoptosis
2.8. In vivo Toxicity Study
2.9. Preparation of Murine CIA Model
2.10. Therapeutic Effect on CIA Model
2.11. Histological Examination
2.12. Data Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of TP-CMCS
3.2. In Vitro Cytotoxicity of TP-CMCS
3.3. TP-CMCS Decreased TP-Induced Apoptosis
3.4. In Vivo Toxicity of TP-CMCS
3.5. Therapeutic Effect on CIA Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pisetsky, D.S. Advances in the Treatment of Rheumatoid Arthritis: Costs and Challenges. N. C. Med. J. 2017, 78, 337–340. [Google Scholar] [PubMed]
- Rahman, M.; Beg, S.; Verma, A.; Al Abbasi, F.A.; Anwar, F.; Saini, S.; Akhter, S.; Kumar, V. Phytoconstituents as pharmacotherapeutics in rheumatoid arthritis: Challenges and scope of nano/submicromedicine in its effective delivery. J. Pharm. Pharmacol. 2017, 69, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanjundaiah, S.M.; Venkatesha, S.H.; Yu, H.; Tong, L.; Stains, J.P.; Moudgil, K.D. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J. Biol. Chem. 2012, 287, 22216–22226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldbach-Mansky, R.; Wilson, M.; Fleischmann, R.; Olsen, N.; Silverfield, J.; Kempf, P.; Kivitz, A.; Sherrer, Y.; Pucino, F.; Csako, G.; et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: A randomized trial. Ann. Intern. Med. 2009, 151, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, L.; Wang, X.; Ren, R.; Wei, X.; Zhao, G.; Yang, J.; Yuan, H.; Pang, H.; Wang, H.; Wang, D. The Development of a Macromolecular Analgesic for Arthritic Pain. Mol. Pharm. 2019, 16, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Zhang, S.N. Herbal compounds for rheumatoid arthritis: Literatures review and cheminformatics prediction. Phytother. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yuan, X.; Pei, Y.H.; Ye, H.Y.; Peng, A.H.; Tang, M.H.; Guo, D.L.; Deng, Y.; Chen, L.J. Anti-inflammatory Ellagitannins from Cleidion brevipetiolatum for the Treatment of Rheumatoid Arthritis. J. Nat. Prod. 2019, 82, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, H.H.; Linghu, K.; Huang, R.Y.; Li, G.; Zuo, H.; Yu, H.; Chan, G.; Hu, Y. Novel Compound-Target Interactions Prediction for the Herbal Formula Hua-Yu-Qiang-Shen-Tong-Bi-Fang. Chem. Pharm. Bull. (Tokyo) 2019, 67, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zheng, Q.; Han, J.; Gao, G.; Liu, J.; Gong, T.; Gu, Z.; Huang, Y.; Sun, X.; He, Q. The targeting of 14-succinate triptolide-lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterials 2009, 30, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, T.; Chen, G.; Li, N.; Wang, J.; Ma, P.; Cao, X. Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-αB activation. Biochem. Biophys. Res. Commun. 2006, 345, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Dai, Y.; Zhao, J.; Lin, L.; Wang, Y.; Wang, Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front. Pharmacol. 2018, 9, 104–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Huang, X.; Wang, D.; Li, M.; Liu, Z. Triptolide protects bone against destruction by targeting RANKL-mediated ERK/AKT signalling pathway in the collagen-induced rheumatoid arthritis. Biomed. Res. 2017, 28, 4111–4116. [Google Scholar]
- Lee, S.Y.; Wee, A.S.; Lim, C.K.; Abbas, A.A.; Selvaratnam, L.; Merican, A.M.; Ahmad, T.S.; Kamarul, T. Supermacroporous poly(vinyl alcohol)-carboxylmethyl chitosan-poly (ethylene glycol) scaffold: An in vitro and in vivo pre-assessments for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Nguyen, T.T.; Nguyen, T.T.; Nguyen, P.P.T.; Nguyen, A.D.; Tran, L.T.; Tran-Van, H. Preparation and in vitro evaluation of FGF-2 incorporated carboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2017, 173, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.M.; Jiang, T.F.; Wang, Y.H.; Wang, D.Y.; Lv, Z.H. Identification and analysis of an impurity inducing clinical adverse effect in anti-adhesion carboxymethyl chitosan products. J. Pharm. Biomed. Anal. 2013, 85, 21–27. [Google Scholar] [CrossRef] [PubMed]
- He, Q.L.; Minn, I.; Wang, Q.; Xu, P.; Head, S.A.; Datan, E.; Yu, B.; Pomper, M.G.; Liu, J.O. Targeted Delivery and Sustained Antitumor Activity of Triptolide through Glucose Conjugation. Angew. Chem. Int. Ed. Engl. 2016, 55, 12035–12039. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, J.S.; Lee, S.J.; Jang, J.; Park, J.S.; Back, S.H.; Bahn, G.; Park, J.H.; Kang, Y.M.; Kim, S.H.; et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J. Control. Release 2015, 216, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Safavy, A.; Georg, G.I.; Vander Velde, D.; Raisch, K.P.; Safavy, K.; Carpenter, M.; Wang, W.; Bonner, J.A.; Khazaeli, M.B.; Buchsbaum, D.J. Site-specifically traced drug release and biodistribution of a paclitaxel-antibody conjugate toward improvement of the linker structure. Bioconjug. Chem. 2004, 15, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, H.; Lee, I.H.; Jon, S. In vivo antitumor effects of chitosan-conjugated docetaxel after oral administration. J. Control. Release 2009, 140, 79–85. [Google Scholar] [CrossRef] [PubMed]












| Exposure Time (h) | IC50 Value (nmol/L) | |
|---|---|---|
| TP | TP-CMCS | |
| 24 | 41.47 ± 8.87 | 221.86 ± 12.87 |
| 48 | 27.14 ± 10.21 | 120.81 ± 21.23 |
| Groups | AST (IU/L) | ALT (IU/L) | BUN (mmol/L) | CRE (μmol/L) |
|---|---|---|---|---|
| Control | 31.80 ± 6.26 | 23.04 ± 2.46 | 18.97 ± 4.82 | 18.86 ± 12.40 |
| TP (0.5mg/kg) | 150.03 ± 54.87 ** | 75.46 ± 28.78 ** | 37.72 ± 4.56 ** | 64.96 ± 34.10 ** |
| TP-CMCS (2mg/kg) | 69.73 ± 27.62 *# | 28.11 ± 2.12 ** ## | 24.71 ± 4.96 * ## | 28.51 ± 12.87 # |
| TP-CMCS (1mg/kg) | 46.56 ± 18.45 ## | 25.96 ± 2.63 * ## | 21.99 ± 5.62 ## | 23.24 ± 15.02 ## |
| TP-CMCS (0.5mg/kg) | 39.06 ± 9.16 ## | 24.39 ± 1.80 ## | 19.87 ± 4.96 ## | 24.77 ± 14.50 ## |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yan, M.; Chen, K.; Tian, Q.; Song, J.; Zhang, Z.; Xie, Z.; Yuan, Y.; Jia, Y.; Zhu, X.; et al. Novel Carboxylated Chitosan-Based Triptolide Conjugate for the Treatment of Rheumatoid Arthritis. Pharmaceutics 2020, 12, 202. https://doi.org/10.3390/pharmaceutics12030202
Zhang L, Yan M, Chen K, Tian Q, Song J, Zhang Z, Xie Z, Yuan Y, Jia Y, Zhu X, et al. Novel Carboxylated Chitosan-Based Triptolide Conjugate for the Treatment of Rheumatoid Arthritis. Pharmaceutics. 2020; 12(3):202. https://doi.org/10.3390/pharmaceutics12030202
Chicago/Turabian StyleZhang, Lan, Min Yan, Kun Chen, Qikang Tian, Junying Song, Zijuan Zhang, Zhishen Xie, Yong Yuan, Yaquan Jia, Xin Zhu, and et al. 2020. "Novel Carboxylated Chitosan-Based Triptolide Conjugate for the Treatment of Rheumatoid Arthritis" Pharmaceutics 12, no. 3: 202. https://doi.org/10.3390/pharmaceutics12030202