1. Introduction
Natural biopolymers, especially polysaccharides, have attracted considerable interest in the field of drug delivery due to their biodegradable, biocompatible, hydrophilic, and protective properties [
1,
2,
3]. In pharmaceutical research and applications, polymeric nano-particles and natural biological macromolecules-based delivery systems are commonly used these days. These potential carriers are capable of delivering bioactive compounds to specific cells and tissues with minimal immune response at the nanoscale. Natural polymers, due to their diverse physico-chemical properties, can be used to fabricate polymeric nano-carrier systems (NCS) that can optimize the bioavailability of drugs by increasing retention time, minimizing side effects, increasing drug solubility, and reducing dosage frequency [
4].
Chitosan, a natural carbohydrate, is inexpensive, easily available, a cationic copolymer, and can be obtained by deacetylation of chitin. It consists of
N-acetyl-
d-glucosamine and
d-glucosamine units. Considering the non-toxic, biocompatible, and biodegradable nature, chitosan is a prospective candidate in the field of pharmaceutical industry [
4,
5]. Several researchers have investigated chitosan in gene delivery, drug release, and biomedical areas [
6,
7,
8,
9]. It can increase the extent of drug delivery across mucosal or nasal layers without causing any damage to the tissues [
10]. On the contrary, various processing conditions (such as a change in temperature, etc.) and environmental factors may trigger degradation and deteriorate its polymeric structure [
11].
It has been observed recently that two or more polymers are usually blended to obtain a wide range of physico-chemical properties. Numerous properties of chitosan can be enhanced by blending with natural polymers as well with synthetic ones such as zein, sodium alginate, curdlan, konjac glucomann, poly(lactic acid), polycaprolactone, poly(ethylene oxide), poly(vinyl pyrollidine), and graphene oxide [
12,
13,
14,
15]. Depending on the nature of biomedical applications, such as tissue engineering and drug delivery, chitosan has been blended with polyethylene glycol fumarate, poly(ethylene glycol) methyl ether, bone, polyethylene glycol, cartilage, skin, gelatin, dialdehyde starch, and nerves [
14,
16,
17,
18,
19,
20].
Poly(allylamine hydrochloride) is a water-soluble weak-base, a cationic and biodegradable polymer. It has a large number of amino groups that are present as a free amine or cationic ammonium salt like other amine group-containing polymers [
21,
22,
23]. Chitosan alone cannot satisfy biomedical applications due to mechanical defects; therefore, blends of chitosan are employed to overcome insufficient mechanical features [
24,
25,
26,
27,
28]. Multitudinous systems comprising of polymeric materials have been prepared to serve as drug carriers to investigate the controlled release behavior of payloads and offer smart drug delivery. Polymeric biocompatible materials such as polyelectrolytes nanoparticles coated with a bilayer of polyelectrolytes, namely PAH and poly (sodium 4-styerenesulfonate) (PSS) were investigated for hydrophobic drug release [
29]. It has been reported that PAH and polyurethane multilayer films as a drug delivery system are potential contestants for pharmaceutical and biomedical applications [
22]. The efficiency of nanoparticulate drug delivery systems can be increased by modifying poly(
d,l-lactide-
co-glycolide) via layer-by-layer adsorption, with two polyelectrolyte pairs such as PAH/PSS and poly(
l-lysine hydrobromide)/dextran sulfate [
30].
Several efforts to develop functional materials (films, beads, and hydrogels) from chitosan have been reported for potential use in wound healing [
31] and drug delivery systems [
32,
33,
34,
35,
36,
37]. Blending of natural polymers with synthetic polymeric material is an alternative approach to incorporate a desired set of physico-mechanical characteristics in new materials. The film matrix generated by the blending of two or more different polymeric systems generally results in modification of physico-mechanical properties, compared to a film obtained by an individual polymer. For instance, chitosan/starch blend films show higher flexibility and improved elongation [
38], chitosan and quaternized poly(4-vinyl-
N-butyl) pyridine demonstrate robust tensile strength [
39], blends of chitosan/poly(vinylalcohol) [
40], chitosan/
N-methylol nylon 6 [
41], and chitosan/polycaprolactone [
12] exhibit improved properties compared to pure chitosan films. Several publications related to the blending of chitosan with natural (particularly cellulose and its derivative) [
42,
43,
44] and synthetic polymers [
11,
41,
45,
46,
47] have been reported.
In continuation of our efforts to develop materials for controlled drug delivery [
48,
49], the purpose of the current study was to develop a smart drug delivery system for ciprofloxacin hydrochloride monohydrate (CPX) using biodegradable CS/PAH blend films. CS and PAH were selected to prepare a series of blend films with different compositions as smart drug delivery matrices. The prepared polymeric films were characterized to explore their properties. To study the drug release behaviour of CS/PAH blend films, CPX was taken as a model drug. CPX is a fluoroquinolone drug that has a broad antibiotic spectrum. It is widely used for microbial infections such as pulmonary, urinary, and dermal infections [
50] and anterior ocular infections [
51]. It can be administered intravenously or orally [
52]. It is very effective against gram-positive and gram-negative bacteria that cause gastrointestinal, urinary tract, abdominal, and respiratory infections. Prophylaxis and osteomyelitis, which is caused by P. aeruginosa, has been treated with CPX [
53]. Due to its low solubility, CPX is usually formulated as a colloidal drug carrier, such as micelles, nano-suspensions, and polymeric nanoparticles [
54]. CPX offers bioavailability (60–80%) and serum half-life (~3–4 h); however, in clinical settings, repeated administration (b.i.d, for 5 days) concerns have made CPX a suitable candidate for controlled-release drug delivery system that can reduce gastric irritation and dose dumping concerns [
55,
56]. A number of studies have shown the incorporation of CPX in polymeric matrices and have demonstrated control release to function as wound-dressing materials [
57,
58,
59], and antibacterial efficacy of CPX-loaded imprinted hydrogels [
60]. To further address the limitations of instantaneous release of CPX, the aim of this study was to design and develop blend films using a biocompatible and biodegradable polymeric blend of CS/PAH in varying compositions, to be used as a drug delivery matrix for sustained release.
We previously reported drug releasing films prepared by blending CS with methoxy polyethylene glycol (mPEG) for controlled drug release applications [
20]. In the present study, based on the well-known fact that cationic polymers have the potential to adhere to negatively charged surfaces of bacteria, we incorporated PAH primarily to impart flexibility in blend films and speculated on the retention of the overall cationic nature of blend films. To the best of our literature survey and knowledge, there are no publications reporting controlled release behaviour of CPX from CS/PAH blended films as potential biomaterials for drug delivery applications.
2. Materials and Methods
2.1. Materials
Chitosan (Cat No. 448869, viscosity: 20–300 cP) with deacetylation degree (DDA) value ~75 to 85% molecular weight: 50 to 190 kDa was purchased from Sigma Aldrich, Saint Louis, MO, USA. Poly(allylamine hydrochloride (PAH) (formula weight: 120,000~200,000 g·mol−1 was obtained from Beantown Chemical, Hudson, NH, USA. Acetic acid (>99.9%) was also purchased from Sigma Aldrich, Saint Louis, MO, USA. Ciprofloxacin hydrochloride monohydrate was collected from Tokyo Chemical Industry Co. Ltd. (TCI, Portland, OR, USA). All of the other chemicals were used as received and without any further purification.
2.2. Preparation of Films
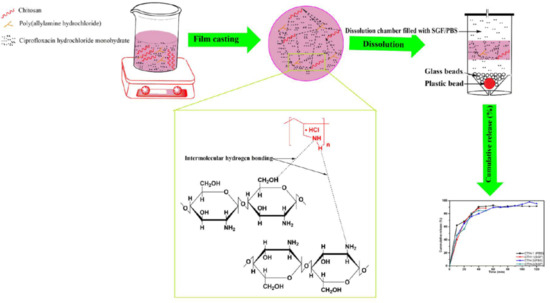
Polymeric blend films of chitosan (CS) with Poly(allylamine hydrochloride) (PAH) were prepared by solution casting technique as reported earlier [
61]. The solutions in different CS/PAH ratios (100/0, 90/10, 80/20, 70/30, 60/40, 40/60) were prepared and each film was coded as CTH-0, CTH-1, CTH-2, CTH-3, CTH-4, CTH-5, respectively. In the first step, chitosan was dissolved in acetic acid solution, 2% (
v/v), with constant stirring for 90 min at room temperature. Secondly, an aqueous solution of PAH, prepared in deionized water, was added in a dropwise manner into the chitosan solutions with continuous stirring at room temperature. These blended solutions were stirred at room temperature for 90 min to obtain homogenous polymeric solutions. For film casting, the polymeric blended solutions were poured into polystyrene petri dishes and allowed to dry under ambient conditions. The blend films were peeled off and immersed in a solution of NaOH (1%), followed by thorough washing with deionized distilled water. Finally, the prepared blended films were placed in an oven at 48 ± 2 °C for 2 days, enclosed in airtight plastic bags, and stored at room temperature until further analysis.
2.3. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
For intermolecular determinations, ATR-FTIR spectra of the prepared films as well as PAH were recorded using a spectrometer (Thermo Electron Corp., Madison, WI, USA). The spectra were taken in transmittance mode ranging from 4000 to 400 cm−1 at a resolution of 4 cm−1 and 256 scans per sample.
2.4. Atomic Force Microscopy (AFM) Studies
Surface characterization of prepared films was subjected to AFM using Nanoscope IIIa Multimode microscope (Veeco Instruments, Inc., Santa Barbara, CA, USA), equipped with silicon RTESP7 cantilever (Veeco Nanoprobe, Camarillo, CA, USA) in tapping mode. For silicon tip, nominal spring constant of 40 N/m was applied. AFM images were recorded as 10 μm × 10 μm using scan rate of 0.5 Hz in air keeping ambient conditions. For AFM, blended solutions of polymers were spin-coated on newly cleaved mica surface using a spin coater (Laurell Technologies Corporation, North Wales, PA, USA). The spinning rate was kept at 1500 RPM for 2 min. All the prepared samples were placed in the oven at 48 ± 2 °C for two days to obtain complete dried films [
13].
2.5. Water Contact Angle (WCA) Measurements
To calculate WCA in degree, the dried films were attached to the microscope glass slides using double-sided tape. A water droplet (8 μL) was placed on each film by a micro-injector syringe (Hamilton Company, Reno, NV, USA). Different images were recorded until WCA was constant using video contact angle instrument VCA optima (AST Products, Inc., Billerica, MA, USA). An automatic WCA measurement, from both the right and left-hand side of the drop, was done using VCA-optima software. On average, twenty measurements were performed per film, which was cut from four different sites.
2.6. Swelling Behaviour of Blend Films
The swelling experiments of pristine CS film and their blend films with PAH were conducted in deionized water and ionic solutions, such as 0.1 M, 0.3 M, 0.5 M, 0.7 M, 0.9 M, 1.0 M NaCl, and CaCl
2 by following previously reported procedure [
62]. All vacuum dried films were cut into smaller pieces, weighed (~20 mg), and dipped into glass vials containing 40 mL of solvent/solution. Every 20 min, excess solvent was discarded, each vial was dried using tissue paper, and the weight of swollen film was measured along with the vial. This procedure was repeated until equilibrium was achieved. The experiment was repeated thrice for each film to calculate the standard deviation (SD). Degree of swelling was calculated using Equation (1):
Wd = weight of the dried films, Ws = weight of the swollen films.
2.7. Cumulative Drug Release (CDR) Study
2.7.1. Preparation of Simulated Gastric Fluid (SGF) and Phosphate Buffer Saline (PBS) Solution
SGF (pH 1.2) was prepared by mixing NaCl (1 g) with 4 mL of HCl and diluted to 500 mL using deionized water [
62]. PBS (pH adjusted to 7.4 using 0.1 M NaOH and 0.1 M HCl) solution was prepared by dissolving NaCl (8 g), KCl (0.2 g), Na
2HPO
4 (1.44 g), and KH
2PO
4 (0.24 g) in 800 mL deionized water and diluted to 1000 mL [
63]. A pH meter (Fischer Scientific accumet, Singapore 139949, Singapore) was used to monitor the pH of solutions.
2.7.2. Drug Loading Method
To study controlled drug release, 20 mg of CPX was dissolved in deionized water. The drug solution was added dropwise in a polymeric blended solution of CS/PAH (90/10). The solution was stirred for 90 min at room temperature prior to pouring into a polystyrene petri dish for film formation under ambient conditions. CPX was also loaded in a film with CS/PAH (80/20) by repeating the same procedure as mentioned above. Finally, the prepared blended films were peeled off, enclosed in airtight plastic bags, and stored at room temperature in the dark until further analysis.
2.7.3. In Vitro Drug Release
The CDR mechanism was studied using a smart dissolution tester (Sortax AG, CH-4123, Allschwil 1, CE 7smart, Basel, Switzerland) [
64]. The average thickness of drug-loaded films was 40–70 µm, determined using a micrometre (Mitutoyo Corporation, model PK-0505CPX, Kanagawa, Japan) by performing three measurements for each specimen. Films were cut from six different regions for triplicate measurements in SGF and PBS solutions. The average weight of the drug-loaded CTH-1 and CTH-2 films was 0.0791 g and 0.0588 g, respectively. These drug-loaded films were put into a 500-mL dissolution medium, such as SGF and PBS solution, at temperature 37 ± 0.5 °C and stirred at 50 rpm. Every 10 min, 5 mL solution was collected separately from each SGF and PBS solution using a pipette. The obtained solutions were filtered through a membrane (pore size; 0.45 μm). Fresh 5 mL of the SGF and PBS solutions were added back every time to keep the volume at 500 mL. The drug release analysis was investigated for 2–3 h in the SGF and PBS solutions. UV scans of the collected solutions were recorded at
λmax 277 nm for SGF and 270 nm for PBS using a UV–Visible spectrometer (Agilent Technologies, Cary 60, UV–Vis, Santa Clara, CA, USA). The standard drug solutions of CPX (100 ppm) in SGF and PBS were used as a reference [
62].
2.8. Statistical Analysis
Data were handled using the SPSS (v.20, IBM, USA) and expressed in the form of means ± standard deviation (SD). The data were analyzed statistically by the two-way analysis of variance (ANOVA) and p < 0.05 was considered as statistically significant. Independent sample t-test was applied to compare drug release in different two media, SGF and PBS.
4. Conclusions
In this study, a drug delivery matrix based on chitosan/PAH blend films with different compositions was successfully prepared by the solution casting method and their physicochemical characteristics were evaluated. These films were found to have a homogenous architecture and excellent surface morphology. AFM exhibited improvements in surface morphology when the PAH concentration was increased up to 60% (w/w). CTH-1 (90% chitosan and 10% PAH) showed maximum WCA, which was 115°. Moreover, the CS/PAH blend films exhibited varied swelling responses in different media and sustained drug release behaviour in PBS, as compared to SGF. The swelling response of blend films in deionized water showed a linear increase as a function of time, and CTH-1 (90% CS and 10% PAH) had a higher degree of swelling, which was around 122 g/g after 80 min, as compared to the other blend films. The maximum observed swelling value in NaCl (0.1 M) for CTH-1 was 41 g/g and in CaCl2 (0.1 M) 31 g/g for CTH-0 (100% CS). Two films were selected, CTH-1 (90% CS and 10% PAH) and CTH-2 (80% CS and 20% PAH), for in vitro CDR at 37 °C. In SGF (pH 1.2), the CDR for CTH-1 and CTH-2 was about 88% and 85% in 50 min, respectively. In SGF, both films were converted into gel-like material after 30 min due to lesser stability of films at lower pH. In the PBS solution (pH 7.4), meanwhile, the CDR for CTH-1 and CTH-2 was around 93% in 90 min and 98% in 120 min, respectively. It can be concluded that the drug was released in a controlled manner in the PBS solution, as compared to the SGF. The selected blend films showed a relatively reduced release (CHT1 = 88% in 50 min, CTH2 = 85% 50 min) in simulated gastric fluid (pH = 1.2) and maximum drug release (CHT1 = 93% in 90 min, CTH2 = 98% 120 min) in phosphate buffer saline (pH = 7.4), thus indicating a pH-responsive (smart) nature. Although the CTH-1 and CTH-2 films showed remarkable drug release, they converted to a gel-like material over a period of time (30 min) in acidic pH (pH = 1.2). This showed the pH-responsive nature of CTH-1 and CTH2. In summary, the results show that chitosan CS/PAH are excellent drug delivery matrices for drug release at pH 7.4. These blend films can be employed for injectable drug delivery systems, tissue regeneration, and associated biomedical applications such as wound dressing due to good compatibility between the drug and the film matrix; they may, however, may not be suitable candidates for oral drug administration.