The Influence of Increasing Concentrations of AMPD on the Efficacy of Its Penetration into a Model Skin Sebum Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Methods
2.2.1. Composition and Preparation of Model Skin Sebum
2.2.2. Determination of Model Skin Sebum Density
2.2.3. Optical Assessment of the Interaction of Alcoholamines with the Components of Model Skin Sebum
2.2.4. Measurement of the Degree of Turbidity of the Evaluated Solution above the Sebum Layer
2.2.5. Measurement of Changes in the pH Value of AMPD Solutions over a Layer of Model Skin Sebum
2.3. Calculation Methods
2.3.1. Calculation of the Penetrated and the Increased Volume of the Sebum Using a Proprietary Computer Program
2.3.2. Calculation of the Volume of Reacted Sebum Based on Measured Changes in the pH Value of a Given Solution.
3. Results
3.1. Initial Assessment of Selected Alcoholamines
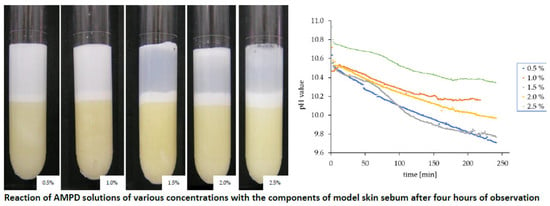
3.2. The Effect of AMPD with the Model Skin Sebum
3.3. Determination of Penetrated and Increased Sebum Volume Based on Observations and Calculations Made Using a Computer Program
3.4. Changes in the pH Value in the AMPD Aqueous Solutions above the Layer of Model Skin Sebum
3.5. Determination of Reacted Volume of Model Skin Sebum Based on the Measurements of Changes in pH of the AMPD Solutions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, S.; Kurokawa, I.; Katoh, N.; Watanabe, K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium aches and Staphylococcus epidermidis isolated from acne lesions. J. Dermatol. 2000, 27, 318–323. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Puhvel, S.M.; Sakamoto, M. Cytotaxin production by comedonal bacteria (Propionibacterium acnes, Propionibacterium granulosum and Staphylococcus epidermidis). J. Investig. Dermatol. 1980, 74, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Gollnick, H.; Schramm, M. Topical drug treatment in acne. Dermatology 1998, 196, 119–125. [Google Scholar] [CrossRef]
- Nix, D.H. Factors to consider when selecting skin cleansing products. J. Wound Ostomy Cont. Nurs. 2000, 27, 260–268. [Google Scholar]
- Solomon, B.A.; Shalita, A.R. Effects of Detergents on Acne. Clin. Dermatol. 1996, 14, 95–99. [Google Scholar] [CrossRef]
- Musial, W.; Kubis, A. Preliminary evaluation of interactions between selected alcoholamines and model skin sebum components. Chem. Pharm. Bull. 2006, 54, 1076–1081. [Google Scholar] [CrossRef] [Green Version]
- Musial, W.; Kubis, A. Preliminary assessment of alginic acid as a factor buffering triethanolamine interacting with artificial skin sebum. Eur. J. Pharm. Biopharm. 2003, 55, 237–240. [Google Scholar] [CrossRef]
- Musial, W.; Kubis, A. Carbopols as factors buffering triethanolamine interacting with artificial skin sebum. Polim. Med. 2004, 34, 17–30. [Google Scholar] [PubMed]
- Final Report on the Safety Assessment of Diisopropanolamine, Triisopropanolamine, Isopropanolamine, and Mixed Isopropanolamine. Int. J. Toxicol. 1987, 6, 53–76.
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Tromethamine, Aminomethyl Propanediol, and Aminoethyl Propanediol as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 5S–18S. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Triethanolamine and Triethanolamine-Containing Ingredients as Used in Cosmetics. Int. J. Toxicol. 2013, 59S–83S. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Van Den Bergh, B. The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem. Phys. Lipids 1998, 91, 85–96. [Google Scholar] [CrossRef]
- Greene, R.S.; Downing, D.T.; Pochi, P.E.; Strauss, J.S. Anatomical variation in the amount and composition of human skin surface lipid. J. Investig. Dermatol. 1970, 54, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downing, D.T.; Stewart, M.E.; Wertz, P.W.; Colton, S.W.; Abraham, W.; Strauss, J.S. Skin lipids: An update. J. Investig. Dermatol. 1987, 88, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Stefaniak, A.B.; Harvey, C.J. Dissolution of materials in artificial skin surface film liquids. Toxicol. In Vitro 2006, 20, 1265–1283. [Google Scholar] [CrossRef]
- International Pharmacopoeia, 9th ed.; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/phint/pdf/b/7.1.3.1.3-Determination-of-mass-density,-relative-density-a_.pdf (accessed on 17 December 2020).
- Burton, J.L. The physical properties of sebum in acne vulgaris. Clin. Sci. 1970, 39, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.W. Turbidity. In U.S. Geological Survey Techniques of Water-Resources Investigations; Book 9; 2005; Chapter A6.7; pp. 1–55. Available online: https://pubs.usgs.gov/twri/twri9a6/twri9a67/twri9a_Section6.7_v2.1.pdf (accessed on 17 December 2020).
- Blanco, Y.S.; Topel, Ö.; Bajnóczi, É.G.; Werner, J.; Björneholm, O.; Persson, I. Chemical equilibria of aqueous ammonium-carboxylate systems in aqueous bulk, close to and at the water-air interface. Phys. Chem. Chem. Phys. 2019, 21, 12434–12445. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Pudney, P.D.A.; Heppenstall-Butler, M.; Butler, M.F.; Ferdinando, D.; Kirkland, M. Interaction of the acid soap of triethanolamine stearate and stearic acid with water. J. Phys. Chem. B 2007, 111, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.P. From pKa to the pH of weak base solutions. Biochem. Mol. Biol. Educ. 2020, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Gordus, A. Chemical equilibrium: IV. Weak acids and bases. J. Chem. Educ. 1999, 68, 397–399. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final amended report on safety assessment on aminomethyl Propanol and aminomethyl propanediol. Int. J. Toxicol. 2009, 28, 141S–161S. [Google Scholar] [CrossRef]
- O’Brien, S.; Kenny, C. The hydrolysis of a salt derived from a weak acid and a weak base. J. Chem. Educ. 1939, 16, 140–142. [Google Scholar] [CrossRef]
- McBay, A. pH approximations. J. Chem. Educ. 1952, 29, 526–529. [Google Scholar] [CrossRef]








| Alcoholamine | AMPD | TRIS | DIPA | TIPA |
|---|---|---|---|---|
| Penetrated volume of model skin sebum, n = 1 (mm3) | 397 | 332 | 357 | 427 |
| Increased volume of model skin sebum over initial level, n = 1 (mm3) | 651 | 813 | 692 | 521 |
| pH value at the beginning of the reaction, n = 1 * | 10.468 ± 0.01 | 10.444 ± 0.01 | 10.708 ± 0.01 | 10.063 ± 0.01 |
| pH value after four hours, n = 1 * | 10.146 ± 0.01 | 9.182 ± 0.01 | 9.853 ± 0.01 | 8.857 ± 0.01 |
| Time [h] | AMPD 0.5% | AMPD 1.0% | AMPD 1.5% | AMPD 2.0% |
|---|---|---|---|---|
| 1 | 0.291 | 0.200 | 0.146 | 0.036 |
| 2 | 0.423 | 0.235 | 0.214 | 0.058 |
| 3 | 0.476 | 0.269 | 0.294 | 0.114 |
| 4 | 0.532 | 0.430 | 0.351 | 0.178 |
| Volume of Reacted Sebum Calculated by Two Methods in 216 min | AMPD 0.5% | AMPD 1.0% | AMPD 1.5% | AMPD 2.0% | AMPD 2.5% |
|---|---|---|---|---|---|
| Penetrated volume of model skin sebum based on observations, n = 4 (mm3) | 226.67 (SD = 112.6) | 233.33 (SD = 61.91) | 285 (SD = 30) | 253.33 (SD = 34.16) | 310.00 (SD = 52.91) |
| Calculated volume of the reacted model skin sebum determined based on measurements of AMPD solution pH changes, n = 1 ** (mm3) | 115.1 (SD = 0.144) | 61.02 (SD = 0.869) | 76.46 (SD = 0.162) | 164.88 (SD = 0.408) | 31.77 (SD = 2.439) |
| difference | 111.57 | 172.31 | 208.54 | 88.45 | 121.77 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzębska, A.; Musiał, W. The Influence of Increasing Concentrations of AMPD on the Efficacy of Its Penetration into a Model Skin Sebum Layer. Pharmaceutics 2020, 12, 1228. https://doi.org/10.3390/pharmaceutics12121228
Kostrzębska A, Musiał W. The Influence of Increasing Concentrations of AMPD on the Efficacy of Its Penetration into a Model Skin Sebum Layer. Pharmaceutics. 2020; 12(12):1228. https://doi.org/10.3390/pharmaceutics12121228
Chicago/Turabian StyleKostrzębska, Agnieszka, and Witold Musiał. 2020. "The Influence of Increasing Concentrations of AMPD on the Efficacy of Its Penetration into a Model Skin Sebum Layer" Pharmaceutics 12, no. 12: 1228. https://doi.org/10.3390/pharmaceutics12121228