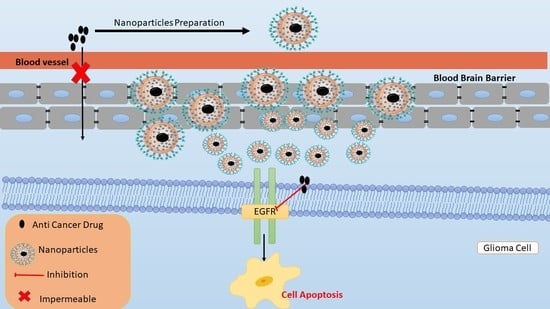
Nanodelivery Systems Targeting Epidermal Growth Factor Receptors for Glioma Management
Abstract
:1. Introduction
2. Molecular Pathology of Glioma
3. Epidemiology and Risk Factors of Glioma
4. Receptors Tyrosine Kinase (RTK) and Their Inhibitors of Intracellular Signaling Pathways in Glioma
4.1. EGFR Family and Its Mutations
4.2. VEGF Family and Its Mutations
5. Molecular Drug Therapy Targets and Its Clinical Profile of EGFR Family in Glioma
5.1. Small-Molecule Kinase Inhibitors
5.2. Targeting Extracellular Domain of RTKs through Antibody Therapies
5.3. Therapies Directed at RTK Ligands
5.4. Targeting Downstream Pathway of EGFR
6. Mechanism of Drug Resistance to EGFR–TKIs in Glioma
7. Current Pharmaceutical Drug Targets in Glioma
7.1. Organic Nanoparticles
7.1.1. Albumin Nanoparticles
7.1.2. Immunoliposomes (IL) and Solid Lipid Nanoparticles (SLNs)
7.1.3. Polymeric Nanoparticles
7.1.4. Dendrimers
7.2. Inorganic Nanoparticles (NPs)
7.2.1. Silica NPs
7.2.2. Magnetic Nanoparticles (MNPs)
7.2.3. Noble Metal Nanoparticles (NM-NPs)
8. Current Clinical Studies of Nanoformulations
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ersoz, M.; Erdemir, A.; Duranoglu, D.; Uzunoglu, D.; Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative evaluation of hesperetin loaded nanoparticles for anticancer activity against C6 glioma cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Patel, A.P.; Fisher, J.L.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Agius, D.; Alahdab, F.; Alam, T.; et al. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñeros, M.; Sierra, M.S.; Izarzugaza, M.I.; Forman, D. Descriptive epidemiology of brain and central nervous system cancers in Central and South America. Cancer Epidemiol. 2016, 44, S141–S149. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Jooma, R.; Waqas, M.; Khan, I. Diffuse low-grade glioma–Changing concepts in diagnosis and management: A review. Asian J. Neurosurg. 2019, 14, 356. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Lahiri, D.; Maji, T.; Biswas, J. Recurrent Glioblastoma: Where we stand. South Asian J. Cancer 2015, 4, 163. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Tom, M.C.; Cahill, D.P.; Buckner, J.C.; Dietrich, J.; Parsons, M.W.; Yu, J.S. Management for Different Glioma Subtypes: Are All Low-Grade Gliomas Created Equal? Am. Soc. Clin. Oncol. Educ. B 2019, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Fung, N.H.; Grima, C.A.; Widodo, S.S.; Kaye, A.H.; Whitehead, C.A.; Stylli, S.S.; Mantamadiotis, T. Understanding and exploiting cell signalling convergence nodes and pathway cross-talk in malignant brain cancer. Cell. Signal. 2019, 57, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Zugazagoitia, J.; Cho, B.; Lovly, C.M.; Paz-Ares, L. Mechanisms of acquired resistance to first-and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018, 29, i10–i19. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S.; et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Yuan, X.; Gao, H.; Yang, Q. Nanoformulations of small molecule protein tyrosine kinases inhibitors potentiate targeted cancer therapy. Int. J. Pharm. 2020, 573, 118785. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef] [Green Version]
- Aum, D.J.; Kim, D.H.; Beaumont, T.L.; Leuthardt, E.C.; Dunn, G.P.; Kim, A.H. Molecular and cellular heterogeneity: The hallmark of glioblastoma. Neurosurg. Focus 2014, 37, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silantyev, A.S.; Falzone, L.; Libra, M.; Gurina, O.I.; Kardashova, K.S.; Nikolouzakis, T.K.; Nosyrev, A.E.; Sutton, C.W.; Mitsias, P.D.; Tsatsakis, A. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells 2019, 8, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelson, N.; Rincon-Torroella, J.; Quiñones-Hinojosa, A.; Greenfield, J.P. Exploring the role of inflammation in the malignant transformation of low-grade gliomas. J. Neuroimmunol. 2016, 297, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kuan, A.S.; Green, J.; Kitahara, C.M.; De González, A.B.; Key, T.; Reeves, G.K.; Floud, S.; Balkwill, A.; Bradbury, K.; Liao, L.M.; et al. Diet and risk of glioma: Combined analysis of 3 large prospective studies in the UK and USA. Neuro-Oncology 2019, 21, 944–952. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Bonavita, E.; Barajon, I.; Garlanda, C.; Mantovani, A.; Jaillon, S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013, 218, 1402–1410. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Powell, D.J. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 2015, 16, 807–820. [Google Scholar] [CrossRef]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-F.; Song, H.-W.; Cai, H.-Q.; Kong, L.-W.; Yao, K.; Jiang, T.; Li, S.-W.; Yan, C.-X.; Wang, P.-F.; Song, H.-W.; et al. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget 2017, 8, 50117–50123. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Yao, X.; Xie, X.; Wu, X.; Zheng, C.; Xia, W.; Ma, S. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J. Urol. 2017, 35, 261–270. [Google Scholar] [CrossRef]
- Xia, W.-K.; Liu, Z.-L.; Shen, D.; Lin, Q.-F.; Su, J.; Mao, W.-D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, F.H.; Atai, N.A.; Gonda, D.; Hughes, M.S.; Mawejje, B.; Balaj, L.; Carter, R.S. Glioma diagnostics and biomarkers: An ongoing challenge in the field of medicine and science. Expert Rev. Mol. Diagn. 2014, 14, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopa, W.; Burley, T.A.; Kramer-Marek, G.; Kaspera, W. Diagnostic and therapeutic biomarkers in glioblastoma: Current status and future perspectives. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-Y.; Zhan, Y.-B.; Zhang, F.-J.; Yu, B.; Ji, Y.-C.; Zhou, J.-Q.; Bai, Y.-H.; Wang, Y.-M.; Wang, L.; Jing, Y.; et al. Prognostic value of preoperative hematological markers combined with molecular pathology in patients with diffuse gliomas. Aging 2019, 11, 6252–6272. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tamimi, A.F.; Juweid, M. Epidemiology and outcome of glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Perkins, A.; Physician, G.L. Primary brain tumors in adults: Diagnosis and treatment. Am. Fam. Physician 2016, 93, 211–217. [Google Scholar]
- Ladomersky, E.; Scholtens, D.M.; Kocherginsky, M.; Hibler, E.A.; Bartom, E.T.; Otto-Meyer, S.; Zhai, L.; Lauing, K.L.; Choi, J.; Sosman, J.A.; et al. The coincidence between increasing age, immunosuppression, and the incidence of patients with glioblastoma. Front. Pharmacol. 2019, 10, 200. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; Zhang, Y. Impact of gender on the survival of patients with glioblastoma. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.S.; Koffie, R.M.; Rattani, A.; Dewan, M.C.; Baticulon, R.E.; Qureshi, M.M.; Wahjoepramono, E.J.; Rosseau, G.; Park, K.; Nahed, B.V. Global incidence of brain and spinal tumors by geographic region and income level based on cancer registry data. J. Clin. Neurosci. 2019, 66, 121–127. [Google Scholar] [CrossRef]
- Gupta, T.; Achari, R.; Chatterjee, A.; Chen, Z.P.; Mehta, M.; Bouffet, E.; Jalali, R. Comparison of epidemiology and outcomes in neuro-oncology between the east and the west: Challenges and opportunities. Clin. Oncol. 2019, 31, 539–548. [Google Scholar] [CrossRef]
- Werlenius, K.; Fekete, B.; Blomstrand, M.; Carén, H.; Jakola, A.S.; Rydenhag, B.; Smits, A. Patterns of care and clinical outcome in assumed glioblastoma without tissue diagnosis: A population-based study of 131 consecutive patients. PLoS ONE 2020, 15, e0228480. [Google Scholar] [CrossRef] [PubMed]
- Bohn, A.; Braley, A.; De La Vega, P.R.; Carlos Zevallos, J.; Barengo, N.C. The association between race and survival in glioblastoma patients in the US: A retrospective cohort study. PLoS ONE 2018, 13, e0198581. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. The 2016 WHO classification of tumours of the central nervous system: The major points of revision. Neurol. Med. Chir. 2017, 57, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. Response to “The epidemiology of glioma in adults: A ‘state of the science’ review”. Neuro-Oncology 2015, 17, 624–626. [Google Scholar] [CrossRef] [Green Version]
- Costanza, M.; Finocchiaro, G. Allergic Signs in Glioma Pathology: Current Knowledge and Future Perspectives. Cancers 2019, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Reblin, M.; Sahebjam, S.; Peeri, N.C.; Martinez, Y.C.; Thompson, Z.; Egan, K.M. Medical cannabis use in glioma patients treated at a comprehensive cancer center in Florida. J. Palliat. Med. 2019, 22, 1202–1207. [Google Scholar] [CrossRef]
- Driver, J.A. Inverse association between cancer and neurodegenerative disease: Review of the epidemiologic and biological evidence. Biogerontology 2014, 15, 547–557. [Google Scholar] [CrossRef]
- Candido, S.; Lupo, G.; Pennisi, M.; Basile, M.S.; Anfuso, C.D.; Petralia, M.C.; Gattuso, G.; Vivarelli, S.; Spandidos, D.A.; Libra, M.; et al. The analysis of miRNA expression profiling datasets reveals inverse microRNA patterns in glioblastoma and Alzheimer’s disease. Oncol. Rep. 2019, 42, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Valle, J.; Tejero, H.; Ibáñez, K.; Portero, J.L.; Krallinger, M.; Al-Shahrour, F.; Tabarés-Seisdedos, R.; Baudot, A.; Valencia, A. A molecular hypothesis to explain direct and inverse co-morbidities between Alzheimer’s Disease, Glioblastoma and Lung cancer. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Regad, T. Targeting RTK signaling pathways in cancer. Cancers 2015, 7, 1758–1784. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Ojo, D.A.; Okeowo, O.T.; Omotuyi, I.O. Exploring receptor tyrosine kinases-inhibitors in Cancer treatments. Egypt. J. Med. Hum. Genet. 2019, 20, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Romano, R.; Bucci, C. Role of EGFR in the Nervous System. Cells 2020, 9, 1887. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Quiñones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell. Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance1. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Xu, Z.G.; Li, S.Q.; He, L.J.; Tang, Y.; Chen, Z.Z.; Yang, D.L. Benzimidazoisoquinoline derivatives inhibit glioblastoma cell proliferation through down-regulating Raf/MEK/ERK and PI3K/AKT pathways. Cancer Cell Int. 2018, 18, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, C.M.; Doherty, G.J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2017, 2, e000279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erfani, P.; Tome-Garcia, J.; Canoll, P.; Doetsch, F.; Tsankova, N.M. EGFR promoter exhibits dynamic histone modifications and binding of ASH2L and P300 in human germinal matrix and gliomas. Epigenetics 2015, 10, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Rutkowska, A.; Stoczyńska-Fidelus, E.; Janik, K.; Włodarczyk, A.; Rieske, P. EGFRvIII: An Oncogene with Ambiguous Role. J. Oncol. 2019, 10922587. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Chekhonin, I.V.; Chekhonin, V.P. The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur. J. Pharmacol. 2017, 810, 70–82. [Google Scholar] [CrossRef]
- Weathers, S.P.; de Groot, J. VEGF manipulation in glioblastoma. Oncology 2015, 29, 720–727. [Google Scholar] [PubMed]
- Soubéran, A.; Brustlein, S.; Gouarné, C.; Chasson, L.; Tchoghandjian, A.; Malissen, M.; Rougon, G. Effects of VEGF blockade on the dynamics of the inflammatory landscape in glioblastoma-bearing mice. J. Neuroinflamm. 2019, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Weihua, Z. Rethink of EGFR in cancer with its kinase independent function on board. Front. Oncol. 2019, 9, 800. [Google Scholar] [CrossRef]
- Kim, Y.; Apetri, M.; Luo, B.B.; Settleman, J.E.; Anderson, K.S. Differential effects of tyrosine kinase inhibitors on normal and oncogenic EGFR signaling and downstream effectors. Mol. Cancer Res. 2015, 13, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Raizer, J.J.; Abrey, L.E.; Lassman, A.B.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Yung, W.K.A.; Gilbert, M.R.; Aldape, K.A.; Wen, P.Y.; et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro-Oncology 2010, 12, 95–103. [Google Scholar] [CrossRef]
- Gallego, O. Nonsurgical treatment of recurrent glioblastoma. Curr. Oncol. 2015, 22, e273–e281. [Google Scholar] [CrossRef] [Green Version]
- Stea, B.; Falsey, R.; Kislin, K.; Patel, J.; Glanzberg, H.; Carey, S.; Ambrad, A.A.; Meuillet, E.J.; Martinez, J.D. Time and dose-dependent radiosensitization of the glioblastoma multiforme U251 cells by the EGF receptor tyrosine kinase inhibitor ZD1839 (‘Iressa’). Cancer Lett. 2003, 202, 43–51. [Google Scholar] [CrossRef]
- Uhm, J.H.; Ballman, K.V.; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II evaluation of gefitinib in patients with newly diagnosed grade 4 astrocytoma: Mayo/north central cancer treatment group study n0074. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Conrad, C.A.; Cloughesy, T.; Prados, M.D.; Friedman, H.S.; Aldape, K.D.; Mischel, P.; Xia, J.; DiLea, C.; Huang, J.; et al. Phase i study of AEE788, a novel multitarget inhibitor of ErbB- and VEGF-receptor-family tyrosine kinases, in recurrent glioblastoma patients. Cancer Chemother. Pharmacol. 2012, 69, 1507–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jane, E.P.; Premkumar, D.R.; Addo-Yobo, S.O.; Pollack, I.F. Abrogation of mitogen-activated protein kinase and akt signaling by vandetanib synergistically potentiates histone deacetylase inhibitor-induced apoptosis in human glioma cells. J. Pharmacol. Exp. Ther. 2009, 331, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Q.; Kaley, T.J.; Duda, D.G.; Schiff, D.; Lassman, A.B.; Wong, E.T.; Mikkelsen, T.; Purow, B.W.; Muzikansky, A.; Ancukiewicz, M.; et al. A multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma patients. Clin. Cancer Res. 2015, 21, 3610–3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiessen, B.; Stewart, C.; Tsao, M.; Kamel-Reid, S.; Schaiquevich, P.; Mason, W.; Easaw, J.; Belanger, K.; Forsyth, P.; McIntosh, L.; et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: Clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother. Pharmacol. 2010, 65, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Schlaff, C.D.; Arscott, W.T.; Gordon, I.; Camphausen, K.A.; Tandle, A. Human EGFR-2, EGFR and HDAC triple-inhibitor CUDC-101 enhances radiosensitiviy of GBM cells. Biomed. Res. J. 2015, 2, 105–119. [Google Scholar]
- Gerstner, E.R.; Eichler, A.F.; Plotkin, S.R.; Drappatz, J.; Doyle, C.L.; Xu, L.; Duda, D.G.; Wen, P.Y.; Jain, R.K.; Batchelor, T.T. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J. Neurooncol. 2011, 103, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Kalpathy-Cramer, J.; Chandra, V.; Da, X.; Ou, Y.; Emblem, K.E.; Muzikansky, A.; Cai, X.; Douw, L.; Evans, J.G.; Dietrich, J.; et al. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J. Neurooncol. 2017, 131, 603–610. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Ervin, T.; Friedman, E.; Priego, V.; Murphy, P.B.; Clark, B.L.; Lamar, R.E. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer 2010, 116, 3663–3669. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [Green Version]
- Fauvel, B.; Yasri, A. Antibodies directed against receptor tyrosine kinases: Current and future strategies to fight cancer. MAbs 2014, 6, 838–851. [Google Scholar] [CrossRef] [Green Version]
- Belda-Iniesta, C.; Carpeño, J.D.C.; Saenz, E.C.; Gutiérrez, M.; Perona, R.; Barón, M.G. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol. Ther. 2006, 5, 912–914. [Google Scholar] [CrossRef] [Green Version]
- Neyns, B.; Sadones, J.; Joosens, E.; Bouttens, F.; Verbeke, L.; Baurain, J.F.; D’Hondt, L.; Strauven, T.; Chaskis, C.; In’t Veld, P.; et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann. Oncol. 2009, 20, 1596–1603. [Google Scholar] [CrossRef]
- Martens, T.; Schmidt, N.O.; Eckerich, C.; Filibrandt, R.; Merchant, M.; Schwall, R.; Westphal, M.; Lamszus, K. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin. Cancer Res. 2006, 12, 6144–6152. [Google Scholar] [CrossRef] [Green Version]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Yu, Y.J.; Watts, R.J. Developing Therapeutic Antibodies for Neurodegenerative Disease. Neurotherapeutics 2013, 10, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Razzak, R.A.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.H.; Kim, M.R.; Jang, J.H.; Na, H.J.; Lee, S. A review of anti-angiogenic targets for monoclonal antibody cancer therapy. Int. J. Mol. Sci. 2017, 18, 1786. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.A.; Aghi, M.K. Bevacizumab for glioblastoma: Current indications, surgical implications, and future directions. Neurosurg. Focus 2014, 37, E9. [Google Scholar] [CrossRef] [Green Version]
- De Groot, J.F.; Lamborn, K.R.; Chang, S.M.; Gilbert, M.R.; Cloughesy, T.F.; Aldape, K.; Yao, J.; Jackson, E.F.; Lieberman, F.; Robins, H.I.; et al. Phase II study of aflibercept in recurrent malignant glioma: A North American brain tumor consortium study. J. Clin. Oncol. 2011, 29, 2689–2695. [Google Scholar] [CrossRef]
- Wang, X.; Yeo, R.X.; Hogg, P.J.; Goldstein, D.; Crowe, P.; Dilda, P.J.; Yang, J.L. The synergistic inhibitory effect of combining therapies targeting EGFR and mitochondria in sarcomas. Oncotarget 2020, 11, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Liu, H.; Ding, Z.B.; Xi, H.P.; Wang, G.W. lncRNA SNHG16 promotes glioma tumorigenicity through miR-373/EGFR axis by activating PI3K/AKT pathway. Genomics 2020, 112, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.F.; Wang, J.; Shao, W.; Wu, C.P.; Chen, Z.P.; To, S.S.T.; Li, W.P. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer 2017, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.R.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Nat. Publ. Gr. 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.J.; Galanis, E.; Anderson, S.K.; Schiff, D.; Kaufmann, T.J.; Peller, P.J.; Giannini, C.; Brown, P.D.; Uhm, J.H.; McGraw, S.; et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro-Oncology 2015, 17, 1261–1269. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Friedman, A.H.; Herndon, J.E.; Marcello, J.; Norfleet, J.A.; McLendon, R.E.; Sampson, J.H.; et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 2010, 96, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.M.; Wen, P.; Cloughesy, T.; Greenberg, H.; Schiff, D.; Conrad, C.; Fink, K.; Robins, H.I.; De Angelis, L.; Raizer, J.; et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Investig. New Drugs 2005, 23, 357–361. [Google Scholar] [CrossRef]
- Kahn, J.; Hayman, T.J.; Jamal, M.; Rath, B.H.; Kramp, T.; Camphausen, K.; Tofilon, P.J. The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro-Oncology 2014, 16, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Buil, L.; Sherris, D.; Beijnen, J.H.; Van Tellingen, O. Dual mTORC1 and mTORC2 inhibitor Palomid 529 penetrates the Blood-Brain Barrier without restriction by ABCB1 and ABCG2. Int. J. Cancer 2013, 133, 1222–1233. [Google Scholar] [CrossRef]
- Huang, L.; Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Bianco, R.; Shin, I.; Ritter, C.A.; Yakes, F.M.; Basso, A.; Rosen, N.; Tsurutani, J.; Dennis, P.A.; Mills, G.B.; Arteaga, C.L. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 2003, 22, 2812–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Q.W.; Cheng, C.K.; Nicolaides, T.P.; Hackett, C.S.; Knight, Z.A.; Shokat, K.M.; Weiss, W.A. A dual phosphoinositide-3-kinase α/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007, 67, 7960–7965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, O.; Cuatrecasas, M.; Benavides, M.; Segura, P.P.; Berrocal, A.; Erill, N.; Colomer, A.; Quintana, M.J.; Balaña, C.; Gil, M.; et al. Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry. J. Neurooncol. 2014, 116, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot, J.F.; Gilbert, M.R.; Aldape, K.; Hess, K.R.; Hanna, T.A.; Ictech, S.; Groves, M.D.; Conrad, C.; Colman, H.; Puduvalli, V.K.; et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J. Neurooncol. 2008, 90, 89–97. [Google Scholar] [CrossRef]
- Akhavan, D.; Pourzia, A.L.; Nourian, A.A.; Williams, K.J.; Nathanson, D.; Babic, I.; Villa, G.R.; Tanaka, K.; Nael, A.; Yang, H.; et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013, 3, 534–547. [Google Scholar] [CrossRef] [Green Version]
- Thorne, A.H.; Zanca, C.; Furnari, F. Epidermal growth factor receptor targeting and challenges in glioblastoma. Neuro-Oncology 2016, 18, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Hegi, M.E.; Stupp, R. Epidermal growth factor receptor inhibitors in neuro-oncology: Hopes and disappointments. Clin. Cancer Res. 2008, 14, 957–960. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Nabors, L.B.; Mason, W.P.; Perry, J.R.; Shapiro, W.; Kavan, P.; Mathieu, D.; Phuphanich, S.; Cseh, A.; Fu, Y.; et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro-Oncology 2015, 17, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Sathornsumetee, S.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Marcello, J.; Herndon, J.E.; Mathe, A.; Hamilton, M.; Rich, J.N.; Norfleet, J.A.; et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro-Oncology 2010, 12, 1300–1310. [Google Scholar] [CrossRef] [Green Version]
- Nam, L.; Coll, C.; Erthal, L.C.S.; de la Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug delivery nanosystems for the localized treatment of glioblastoma multiforme. Materials 2018, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, B.S.; AlAmri, A.H.; McConville, C. Polymeric nanoparticles for the treatment of malignant gliomas. Cancers 2020, 12, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, M.; Maire, C.L.; Lamszus, K. EGFR as a Target for glioblastoma treatment: An unfulfilled promise. CNS Drugs 2017, 31, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [Green Version]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Sun, T.; et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef]
- Li, C.; Tan, J.; Chang, J.; Li, W.; Liu, Z.; Li, N.; Ji, Y. Radioiodine-labeled anti-epidermal growth factor receptor binding bovine serum albumin-polycaprolactone for targeting imaging of glioblastoma. Oncol. Rep. 2017, 38, 2919–2926. [Google Scholar] [CrossRef]
- Tsutsui, Y.; Tomizawa, K.; Nagita, M.; Michiue, H.; Nishiki, T.I.; Ohmori, I.; Seno, M.; Matsui, H. Development of bionanocapsules targeting brain tumors. J. Control. Release 2007, 122, 159–164. [Google Scholar] [CrossRef]
- Wei, L.; Guo, X.Y.; Yang, T.; Yu, M.Z.; Chen, D.W.; Wang, J.C. Brain tumor-targeted therapy by systemic delivery of siRNA with Transferrin receptor-mediated core-shell nanoparticles. Int. J. Pharm. 2016, 510, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; Vijaykumar, V.E.; Natarajan, S.K.; Sengupta, S.; Sabbisetti, V.S. Sustained inhibition of cMET-VEGFR2 signaling using liposome-mediated delivery increases efficacy and reduces toxicity in kidney cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Li, Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G.; Hao, J. Co-delivery of HIF1α siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lee, C.H. Dual targeting of solid lipid nanoparticles grafted with 83-14 MAb and anti-EGF receptor for malignant brain tumor therapy. Life Sci. 2016, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, G.; Yang, W.; Barth, R.F.; Tjarks, W.; Lee, R.J. Synthesis of cetuximab-immunoliposomes via a cholesterol-based membrane anchor for targeting of EGFR. Bioconjug. Chem. 2007, 18, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Weng, K.C.; Hashizume, R.; Noble, C.O.; Serwer, L.P.; Drummond, D.C.; Kirpotin, D.B.; Kuwabara, A.M.; Chao, L.X.; Chen, F.F.; James, C.D.; et al. Convection-enhanced delivery of targeted quantum dot-immunoliposome hybrid nanoparticles to intracranial brain tumor models. Nanomedicine 2013, 8, 1913–1925. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, S.J.; Kim, J.S.; Ryu, J.H.; Kim, J.S. Synergistic effect of immunoliposomal gemcitabine and bevacizumab in glioblastoma stem cell-targeted therapy. J. Biomed. Nanotechnol. 2015, 11, 1989–2002. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.Y.; Han, C.; Yu, L.; Yang, Y.; et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Alves Rico, S.R.; Abbasi, A.Z.; Ribeiro, G.; Ahmed, T.; Wu, X.Y.; De Oliveira Silva, D. Diruthenium (II, III) metallodrugs of ibuprofen and naproxen encapsulated in intravenously injectable polymer-lipid nanoparticles exhibit enhanced activity against breast and prostate cancer cells. Nanoscale 2017, 9, 10701–10714. [Google Scholar] [CrossRef]
- Ni, X.L.; Chen, L.X.; Zhang, H.; Yang, B.; Xu, S.; Wu, M.; Liu, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; et al. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017, 24, 1501–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cheng, P.; Chen, P.; Pu, K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018, 6, 746–765. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagn. Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, Y.; Li, H.; Zhao, Y.; Meng, D.; Li, D.; Shi, J.; Zhang, H.; Zhang, Z.; Zhang, Y. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: Angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J. Drug Target. 2015, 23, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Pan, B.; Xu, H.; Zhao, Z.; Shen, J.; Lu, J.; Yu, R.; Liu, H. Co-delivery of GOLPH3 siRNA and gefitinib by cationic lipid-PLGA nanoparticles improves EGFR-targeted therapy for glioma. J. Mol. Med. 2019, 97, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Duwa, R.; Banstola, A.; Emami, F.; Jeong, J.H.; Lee, S.; Yook, S. Cetuximab conjugated temozolomide-loaded poly (lactic-co-glycolic acid) nanoparticles for targeted nanomedicine in EGFR overexpressing cancer cells. J. Drug Deliv. Sci. Technol. 2020, 60, 101928. [Google Scholar] [CrossRef]
- Yu, F.; Asghar, S.; Zhang, M.; Zhang, J.; Ping, Q.; Xiao, Y. Local strategies and delivery systems for the treatment of malignant gliomas. J. Drug Target. 2019, 27, 367–378. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Márquez-Miranda, V.; Camarada, M.B.; Araya-Durán, I.; Varas-Concha, I.; Almonacid, D.E.; González-Nilo, F.D. Biomimetics: From Bioinformatics to Rational Design of Dendrimers as Gene Carriers. PLoS ONE 2015, 10, e0138392. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Iyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.; Yuan, X.; Li, F.; Pu, P.; Yu, S.; Shen, C.; Zhang, Z.; Zhang, Y. Evaluation of folate-PAMAM for the delivery of antisense oligonucleotides to rat C6 glioma cells in vitro and in vivo. J. Biomed. Mater. Res. Part A 2010, 93, 585–594. [Google Scholar] [CrossRef]
- Wu, G.; Barth, R.F.; Yang, W.; Kawabata, S.; Zhang, L.; Green-Church, K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol. Cancer Ther. 2006, 5, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Gajbhiye, V.; Jain, N.K. The treatment of Glioblastoma Xenografts by surfactant conjugated dendritic nanoconjugates. Biomaterials 2011, 32, 6213–6225. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Research paper enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly(Amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics 2019, 3, 311–330. [Google Scholar] [CrossRef]
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.H.; Zhang, L.M.; Chen, Q.T.; Zhang, Y.; Zhang, Z.J. Synthesis of a novel magnetic drug delivery system composed of doxorubicin-conjugated Fe3O4 nanoparticle cores and a PEG-functionalized porous silica shell. Chem. Commun. 2010, 46, 8633–8635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.Q.; Zheng, Z.; Huang, Z.; Li, Z.; Niu, S.; Liu, J.M. Powerful inner/outer controlled multi-target magnetic nanoparticle drug carrier prepared by liquid photo-immobilization. Sci. Rep. 2014, 4, 4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heggannavar, G.B.; Hiremath, C.G.; Achari, D.D.; Pangarkar, V.G.; Kariduraganavar, M.Y. Development of doxorubicin-loaded magnetic silica-pluronic F-127 nanocarriers conjugated with transferrin for treating glioblastoma across the blood-brain barrier using an in vitro model. ACS Omega 2018, 3, 8017–8026. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.A.; Palankar, R.; Fritsch, C.; Klaver, A.; Kanger, J.S.; Jovin, T.M.; Arndt-Jovin, D.J. Correction: Magnetic nanoparticles as mediators of ligand-free activation of EGFR signaling. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted therapy of glioblastoma stem-like cells and tumor nonstem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef]
- Liu, X.; Du, C.; Li, H.; Jiang, T.; Luo, Z.; Pang, Z.; Geng, D.; Zhang, J. Engineered superparamagnetic iron oxide nanoparticles (SPIONs) for dual-modality imaging of intracranial glioblastoma via EGFRvIII targeting. Beilstein J. Nanotechnol. 2019, 10, 1860–1872. [Google Scholar] [CrossRef]
- Hamarat Şanlıer, Ş.; Ak, G.; Yılmaz, H.; Ünal, A.; Bozkaya, Ü.F.; Tanıyan, G.; Yıldırım, Y.; Yıldız Türkyılmaz, G. Development of ultrasound-triggered and magnetic-targeted nanobubble system for dual-drug delivery. J. Pharm. Sci. 2019, 108, 1272–1283. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Cai, Z.; Kwon, Y.L.; Lechtman, E.; Pignol, J.P.; Reilly, R.M. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res. Treat. 2013, 137, 81–91. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, E.; Lindner, W.; Lämmerhofer, M. Gold nanoparticle-antibody conjugates for specific extraction and subsequent analysis by liquid chromatography-tandem mass spectrometry of malondialdehyde-modified low density lipoprotein as biomarker for cardiovascular risk. Anal. Chim. Acta 2015, 857, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Cheng, Y.; Chang, Y.; Jian, H.; Zheng, R.; Wu, X.; Xu, K.; Wang, L.; Ma, X.; Li, X.; et al. Time-staggered delivery of erlotinib and doxorubicin by gold nanocages with two smart polymers for reprogrammable release and synergistic with photothermal therapy. Biomaterials 2019, 217, 119327. [Google Scholar] [CrossRef]
- Groysbeck, N.; Stoessel, A.; Donzeau, M.; Da Silva, E.C.; Lehmann, M.; Strub, J.M.; Cianferani, S.; Dembélé, K.; Zuber, G. Synthesis and biological evaluation of 2.4 nm thiolate-protected gold nanoparticles conjugated to Cetuximab for targeting glioblastoma cancer cells via the EGFR. Nanotechnology 2019, 30, 184005. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Kwan, K.; Schneider, J.R.; Kobets, A.; Boockvar, J.A. Targeting epidermal growth factor receptors in recurrent glioblastoma via a novel epithelial growth factor receptor-conjugated nanocell doxorubicin delivery system. Clin. Neurosurg. 2018, 82, N23–N24. [Google Scholar] [CrossRef] [Green Version]
- Mamot, C.; Ritschard, R.; Wicki, A.; Stehle, G.; Dieterle, T.; Bubendorf, L.; Hilker, C.; Deuster, S.; Herrmann, R.; Rochlitz, C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: A phase 1 dose-escalation study. Lancet Oncol. 2012, 13, 1234–1241. [Google Scholar] [CrossRef]
- Senzer, N.; Nemunaitis, J.; Nemunaitis, D.; Bedell, C.; Edelman, G.; Barve, M.; Nunan, R.; Pirollo, K.F.; Rait, A.; Chang, E.H. Phase i study of a systemically delivered p53 nanoparticle in advanced solid tumors. In Proceedings of the Molecular Therapy; Nature Publishing Group: Edinburgh, UK, 2013; pp. 1096–1103. [Google Scholar]
- Kesari, S.; Juarez, T.; Carrillo, J.; Truong, J.; Nguyen, M.; Heng, A.; Gill, J.; Nguyen, H.; Nomura, N.; Grigorian, B.; et al. A phase 2 trial with Abi-009 (nab-sirolimus) as single-agent and combinations in recurrent high-grade glioma (RHGG) and in newly diagnosed glioblastoma (ndGBM). Neuro-Oncology 2019, 21, vi218–vi219. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Rajakani, M. Problems in cryptography and cryptanalysis. In Algorithmic Strategies for Solving Complex Problems in Cryptography; IGI Global: Hershey, PA, USA, 2017; pp. 23–38. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Stompor, M.; Podgórski, R.; Kubrak, T. Combined effect of flavonoid compounds and cytostatics in cancer treatment. Eur. J. Clin. Exp. Med. 2017, 15, 157–164. [Google Scholar] [CrossRef]
- Sharma, N.; Dobhal, M.; Joshi, Y.; Chahar, M. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, H.S.; Xu, R.; Won, J.Y.; Chin, Y.W.; Yim, H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int. J. Mol. Sci. 2019, 20, 2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, V.; Herrera, F.; García-Santos, G.; Antolín, I.; Rodriguez-Blanco, J.; Rodriguez, C. Signaling pathways involved in antioxidant control of glioma cell proliferation. Free Radic. Biol. Med. 2007, 42, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The role of JAK-STAT signaling within the CNS. Jak-Stat 2013, 2, e22925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiatek-Machado, K.; Kaminska, B. STAT Signaling in Glioma Cells. Adv. Exp. Med. Biol. 2013, 1202, 189–208. [Google Scholar] [CrossRef]







| Drugs | IC50 (nmol/L) | Targeting Receptor | Disease |
|---|---|---|---|
| Gefitinib (Iressa®) | 14.6 | EGFR | NSCLC, pancreatic cancer |
| Erlotinib (Tarceva®) | 2 | ||
| Icotinib (Conmana®) | 45 | ||
| Lapatinib (Tykerb®) | 10.8, 9.2 | EGFR, HER2 | Breast cancer |
| Neratinib (Nerlynx®) | 92, 59 | EGFR, HER2 | NSCLC, breast cancer |
| Afatinib (Gilotrif®) | 0.5 | EGFR, HER2 14 | NSCLC, breast cancer, squamous cell carcinoma of the head and neck |
| Imatinib (Glivec®, Gleevec®) | 600, 100 | Abl, PDGFR, Kit SRC, PDGFR | CML, CMML, GIST |
| Dasatinib (Sprycel®) | <10 | CML resistant to imatinib | |
| Nilotinib (Tasigna®) | <30 | CML resistant to imatinib | |
| Sunitinib (Sutent®) | <100 | VEGFR1-3, PDGFR, Kit FLT3, RET, CSF1R GIST, BRAF | Advanced RCC, CML resistant to imatinib, Hepatocellular carcinoma |
| Sorafenib (Nexavar®) | <100 | ||
| Pazopanib (Votrient®) | <150 |
| Drug | GBM | Phase | Characteristics | NCT No. |
|---|---|---|---|---|
| Small-Molecule Kinase Inhibitors and/or Combination with Other Therapy | ||||
| GC1118 | R | II | Focuses on overall response rate and exploration of predictive/prognostic biomarkers | NCT03618667 |
| Osimertinib Fludeoxyglucose F-18 (FDG) | R | II | Studied the intra-patient variability of tumor FDG uptake, which was determined using double baseline FDG PET prior to osimertinib exposure | NCT03732352 |
| EGFR BATs with SOC RT and TMZ | R | I | Immune measures in blood anti-GBM cytotoxicity of peripheral blood mononuclear cells directed at GBM cell lines | NCT03344250 |
| Dacomitinib | C | II | Progression-free survival (PFS) at six months (PFS6m) and Safety and tolerability of oral administration of PF-00299804. | NCT01520870 |
| Temozolomide, ABT-414, Radiation | A, NR | II III | Overall Survival (OS) | NCT02573324 |
| EGFR(V)-EDV-Dox | R | I | Overall Survival (OS) and identification of recommended phase 2 dose of EGFR(V)-EDV-Dox in subjects with recurrent GBM | NCT02766699 |
| C225-ILs-dox | R | I | Tumor response achieved in the treatment phase was assessed as per RANO criteria | NCT03603379 |
| Protein expression analysis | C | - | Overall survival and the free survival was predicted based on the molecular characteristics | NCT00897663 |
| EGFRvIII-CARs | R | I | Assessment of T cell trafficking within the brain tumor | NCT03283631 |
| EGFRBi Armed Autologous T Cells | W | I II | Overall survival, change of cytokine profile, incident toxicity, and the overall survival was assessed as per the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0 | NCT02521090 |
| Erlotinib hydrochloride | T | II | Disease response measured objectively by MRI of brain duration of progression-free survival (PFS) | NCT00387894 |
| Radiation, temozolomide depatuxizumab mafodotin | A, NR | III | Cumulative dose of depatuxizumab mafodotin | NCT03419403 |
| Gefitinib + Radiation therapy | C | I II | Overall survival by EGFR status | NCT00052208 |
| Cetuximab, Mannitol | R | I II | Composite overall response rate was assessed through RANO | NCT02861898 |
| AMG 596 | R | I | Number of subject with treatment-emergent adverse events | NCT03296696 |
| AMG 596 | C | I | Overall survival and anti-AMG 595 antibody formation | NCT01475006 |
| PI3K/ART/mTOR | ||||
| PX-866 | C | II | Measurement of progression and response of brain tumor using MRI or CT scan | NCT01259869 |
| INC280 | T | I, II | Number of Patients Reporting Dose Limiting Toxicities (DLTs) in Phase 1 and Phase II Surgical Arm: Concentrations of INC280 and Buparlisib in Tumor | NCT01870726 |
| XL765 (SAR 245409) + XL147 (SAR 245408) | C | I | To assess the biological effect and PI3K/mTOR modulations of XL 765 and XL 147 in GBM tissue | NCT01240460 |
| BKM120 + Surgery | C | II | BKM120 brain plasma ratio at time of surgery | NCT01339052 |
| MK-3475 + PI3K/AKT Inhibitors | # | I, II | Progression-free survival | NCT02430363 |
| GDC-0084 + Radio Therapy | R | I | To estimate the maximum tolerated dose (MTD) or RP2D of GDC-0084 after radiation therapy (RT) | NCT03696355 |
| AZD2014 | A, NR | I | Recommended phase II dose (RP2D) of AZD2014 | NCT02619864 |
| GDC-0084 | R | II | Dose-limiting toxicities (DLTs) | NCT03522298 |
| AZD8055 | C | I | Establishment of MTD of AZD8055 with recurrent gliomas | NCT01316809 |
| GDC-0084 + Radiation Therapy | R | I | To estimate the MTD or RP2D of GDC-0084 after RT | NCT03696355 |
| CC-115 | A, NR | I | To determine the MTD, Non-tolerated dose and Dose-Limiting Toxicity | NCT01353625 |
| Nano Carriers | Drug | Phase | Outcome Measures | NCT Number | Reference |
|---|---|---|---|---|---|
| EnGeneIC delivery vehicle (EDV) | EGFR-EDV-DOX | I | Determination of a possible phase II dose of drug for recurrent GBM. | NCT02766699 | [168] |
| ILs | C225-IL-DOX | I | Determination of a suitable ratio of C225–IL–DOX concentration. | NCT03603379 | [169] |
| PEGylated Lipososmes | DOX-Trastuzumab | I/II | To determine the safety and tolerability of i.v. administration of the PEGylated liposomes | NCT01386580 | NA |
| Albumin NPs | Rapamycin + Avastin + Radiation | II | To determine progression-free survival (PFS) and overall survival (OS) rate according to response assessment in neuro-oncology (RANO) criteria | NCT03463265 | [171] |
| Cationic Lipososmes | SGT-53 + TMZ | II | To determine six-month PFS and OS, anti-cancer activity, safety, and efficacy of NPs. | NCT02340156 | [170] |
| enzyme-linked immune spots | EGFR-Bi-T | I/II | To determine the maximum tolerated dose (MTD) for eight intrathecal (IT) injections | NCT02521090 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paranthaman, S.; Goravinahalli Shivananjegowda, M.; Mahadev, M.; Moin, A.; Hagalavadi Nanjappa, S.; Nanjaiyah, N.D.; Chidambaram, S.B.; Gowda, D.V. Nanodelivery Systems Targeting Epidermal Growth Factor Receptors for Glioma Management. Pharmaceutics 2020, 12, 1198. https://doi.org/10.3390/pharmaceutics12121198
Paranthaman S, Goravinahalli Shivananjegowda M, Mahadev M, Moin A, Hagalavadi Nanjappa S, Nanjaiyah ND, Chidambaram SB, Gowda DV. Nanodelivery Systems Targeting Epidermal Growth Factor Receptors for Glioma Management. Pharmaceutics. 2020; 12(12):1198. https://doi.org/10.3390/pharmaceutics12121198
Chicago/Turabian StyleParanthaman, Sathishbabu, Meghana Goravinahalli Shivananjegowda, Manohar Mahadev, Afrasim Moin, Shivakumar Hagalavadi Nanjappa, Nandakumar Dalavaikodihalli Nanjaiyah, Saravana Babu Chidambaram, and Devegowda Vishakante Gowda. 2020. "Nanodelivery Systems Targeting Epidermal Growth Factor Receptors for Glioma Management" Pharmaceutics 12, no. 12: 1198. https://doi.org/10.3390/pharmaceutics12121198