Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles
Abstract
:1. Introduction
2. Ratiometric Drug Delivery
2.1. Release of Co-Loaded Drugs through pH Control
2.2. Release of Co-Loaded Drugs through Polymeric Degradation
2.3. The Release of Co-Loaded Drugs through Enzymatic Degradation
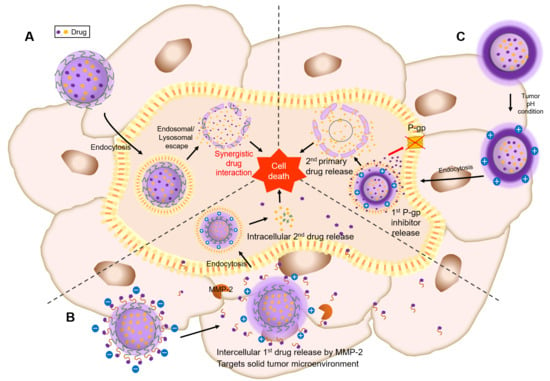
3. Sequential Drug Release
3.1. Intracellular Sequential Drug Release
3.1.1. Sequential Drug Release of Co-Loaded Drugs That Directly Affect Cancer Cells
3.1.2. Sequential Drug Release Where One of the Co-Loaded Drugs Amplifies the Effect of the Other Drug
3.2. Sequential Drug Release to Achieve both Intercellular and Intracellular (Spatiotemporal) Drug Release
3.2.1. Spatiotemporal Drugs Release by Matrix Metalloproteinase-2 (MMP-2)
3.2.2. Spatiotemporal Drugs Release by Dual-pH-Responsive Nanocarriers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.H.; Kwon, G.S. Epothilone B-based 3-in-1 polymeric micelle for anticancer drug therapy. Int. J. Pharm. 2017, 518, 307–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheff, R.J.; Schneider, B.J. Non-small-cell lung cancer: Treatment of late stage disease: Chemotherapeutics and new frontiers. Semin. Interv. Radiol. 2013, 30, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Lu, Y.; Xie, J.-L.; Gao, Z.-K.; Wu, X.-B.; Yao, W.; Gu, W. Overexpression of miR-758 inhibited proliferation, migration, invasion, and promoted apoptosis of non-small cell lung cancer cells by negatively regulating HMGB. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef] [Green Version]
- Ascierto, P.A.; Marincola, F.M. Combination Therapy: The Next Opportunity and Challenge of Medicine; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lee, J.H.; Nan, A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Giaccone, G.; Pinedo, H.M. Drug resistance. Oncologist 1996, 1, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Kohli, M.; Smith, A. Nanoparticles for Combination Drug Therapy. ACS Nano 2013, 7, 9518–9525. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today 2012, 17, 1044–1052. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.D. A review on recent advancement of cancer therapy using nanoparticles. Biochem. Mol. Biol. Lett. 2017, 3, 104. [Google Scholar]
- Solanki, N.; Mehta, M.; Chellappan, D.K.; Gupta, G.; Hansbro, N.G.; Tambuwala, M.M.; Aa Aljabali, A.; Paudel, K.R.; Liu, G.; Satija, S.; et al. Antiproliferative effects of boswellic acid-loaded chitosan nanoparticles on human lung cancer cell line A549. Future Med. Chem. 2020, 12, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Sun, Y.; Li, Y.; Hao, F.; Qiu, P.; Teng, L.; Xie, J.; Gao, Y. Docetaxel-loaded human serum albumin (HSA) nanoparticles: Synthesis, characterization, and evaluation. Biomed. Eng. Online 2019, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Al Zoubi, M.S.; Al-Trad, B.M.; Nasef, M.; Satija, S.; Mehta, M. Albumin nano-encapsulation of piceatannol enhances its anticancer potential in colon cancer via downregulation of nuclear p65 and HIF-1α. Cancers 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, M.J.; Jo, Y.H.; Lee, Y.J.; Park, C.W.; Kim, J.S.; Hong, J.T.; Chung, Y.B.; Lee, M.K.; Shin, D.H. Physicochemical, Pharmacokinetic, and Toxicity Evaluation of Methoxy Poly(ethylene glycol)-b-Poly(d,l-Lactide) Polymeric Micelles Encapsulating Alpinumisoflavone Extracted from Unripe Cudrania tricuspidata Fruit. Pharmaceutics 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2014, 3, 111–122. [Google Scholar] [CrossRef]
- Wakaskar, R.R. Passive and active targeting in tumor microenvironment. Int. J. Drug Dev. Res. 2017, 9, 37–41. [Google Scholar]
- Kolishetti, N.; Dhar, S.; Valencia, P.M.; Lin, L.Q.; Karnik, R.; Lippard, S.J.; Langer, R.; Farokhzad, O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Zhang, L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef]
- Yan, Y.; Björnmalm, M.; Caruso, F. Particle Carriers for Combating Multidrug-Resistant Cancer. ACS Nano 2013, 7, 9512–9517. [Google Scholar] [CrossRef] [PubMed]
- Batist, G.; Gelmon, K.A.; Chi, K.N.; Miller, W.H.; Chia, S.K.; Mayer, L.D.; Swenson, C.E.; Janoff, A.S.; Louie, A.C. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin. Cancer Res. 2009, 15, 692–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, E.J.; Lancet, J.E.; Kolitz, J.E.; Ritchie, E.K.; Roboz, G.J.; List, A.F.; Allen, S.L.; Asatiani, E.; Mayer, L.D.; Swenson, C. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5: 1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenstein, J.R.; Shin, H.-C.; Kasmerchak, K.; Buehler, D.; Kwon, G.S.; Kozak, K.R. Antitumor activity of Triolimus: A novel multidrug-loaded micelle containing Paclitaxel, Rapamycin, and 17-AAG. Mol. Cancer Ther. 2012, 11, 2233–2242. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-C.; Alani, A.W.; Cho, H.; Bae, Y.; Kolesar, J.M.; Kwon, G.S. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol. Pharm. 2011, 8, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-C.; Cho, H.; Lai, T.C.; Kozak, K.R.; Kolesar, J.M.; Kwon, G.S. Pharmacokinetic study of 3-in-1 poly(ethylene glycol)-block-poly(D, L-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin. J. Control. Release 2012, 163, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.L.; Fan, Z.L.; ZhuGe, D.L.; Tong, M.Q.; Shen, B.X.; Lin, M.T.; Zhu, Q.Y.; Jin, B.H.; Sohawon, Y.; Yao, Q.; et al. Ratiometric delivery of two therapeutic candidates with inherently dissimilar physicochemical property through pH-sensitive core-shell nanoparticles targeting the heterogeneous tumor cells of glioma. Drug Deliv. 2018, 25, 1302–1318. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Sui, J.; Ma, M.; Hu, J.; Sun, Y.; Yang, L.; Fan, Y.; Zhang, X. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J. Control. Release Off. J. Control. Release Soc. 2020, 326, 350–364. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Zhang, J.; Kim, W.Y.; Huang, L. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv. Funct. Mater. 2014, 24, 6601–6611. [Google Scholar] [CrossRef]
- Guo, S.; Lin, C.M.; Xu, Z.; Miao, L.; Wang, Y.; Huang, L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano 2014, 8, 4996–5009. [Google Scholar] [CrossRef]
- Luo, S.; Gu, Y.; Zhang, Y.; Guo, P.; Mukerabigwi, J.F.; Liu, M.; Lei, S.; Cao, Y.; He, H.; Huang, X. Precise Ratiometric Control of Dual Drugs through a Single Macromolecule for Combination Therapy. Mol. Pharm. 2015, 12, 2318–2327. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Jeena, M.T.; Kim, K.; Yong Oh, J.; Kim, C.; Park, M.-H.; Ryu, J.-H. Spatiotemporally and Sequentially-Controlled Drug Release from Polymer Gatekeeper–Hollow Silica Nanoparticles. Sci. Rep. 2017, 7, 46540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, M.; Zhou, L.; Han, Q.; Chen, X.; Li, S.; Li, L.; Su, Z.; Wang, C. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials 2018, 181, 113–125. [Google Scholar] [CrossRef]
- Ye, M.; Han, Y.; Tang, J.; Piao, Y.; Liu, X.; Zhou, Z.; Gao, J.; Rao, J.; Shen, Y. A Tumor-Specific Cascade Amplification Drug Release Nanoparticle for Overcoming Multidrug Resistance in Cancers. Adv. Mater. 2017, 29, 1702342. [Google Scholar] [CrossRef] [PubMed]
- Luan, T.; Cheng, L.; Cheng, J.; Zhang, X.; Cao, Y.; Zhang, X.; Cui, H.; Zhao, G. Tailored Design of an ROS-Responsive Drug Release Platform for Enhanced Tumor Therapy via “Sequential Induced Activation Processes”. Acs Appl. Mater. Interfaces 2019, 11, 25654–25663. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, B.; Ye, H.; Zhang, X.; Song, H.; Wang, X.; Li, N.; Wei, L.; Wang, Y.; Zhang, H.; et al. Self-Strengthened Oxidation-Responsive Bioactivating Prodrug Nanosystem with Sequential and Synergistically Facilitated Drug Release for Treatment of Breast Cancer. ACS Appl. Mater. Interfaces 2019, 11, 18914–18922. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, Y.; Xiao, H.; Xiao, Z.; Guo, Y.; Cheng, D.; Shuai, X. Core–Shell Distinct Nanodrug Showing On-Demand Sequential Drug Release To Act on Multiple Cell Types for Synergistic Anticancer Therapy. ACS Nano 2019, 13, 7036–7049. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xiao, H.; Li, B.; Peng, Y.; Li, X.; Wang, Y.; Adamus, G.; Kowalczuk, M.; Shuai, X. The programmed site-specific delivery of the angiostatin sunitinib and chemotherapeutic paclitaxel for highly efficient tumor treatment. J. Mater. Chem. B 2019, 7, 4953–4962. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Liu, H.; Wang, T.; Tang, S.; Zhang, J.; Zhang, X. Site-specific drug-releasing polypeptide nanocarriers based on dual-pH response for enhanced therapeutic efficacy against drug-resistant tumors. Theranostics 2015, 5, 890. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy—Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Mayer, L.D.; Janoff, A.S. Optimizing combination chemotherapy by controlling drug ratios. Mol. Interv. 2007, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Grantab, R.; Sivananthan, S.; Tannock, I.F. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 2006, 66, 1033–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, N.; Tang, B.; Liu, G.; Liang, X. Poly (γ-glutamic acid)-coated lipoplexes loaded with Doxorubicin for enhancing the antitumor activity against liver tumors. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanomedicine: A road to cancer therapeutics. Curr. Pharm. Des. 2013, 19, 1994–2010. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef]
- Jin, Q.; Deng, Y.; Chen, X.; Ji, J. Rational Design of Cancer Nanomedicine for Simultaneous Stealth Surface and Enhanced Cellular Uptake. ACS Nano 2019, 13, 954–977. [Google Scholar] [CrossRef]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane Phosphatidylserine Regulates Surface Charge and Protein Localization. Science 2008, 319, 210. [Google Scholar] [CrossRef]
- Du, J.Z.; Du, X.J.; Mao, C.Q.; Wang, J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Cui, J.; Yan, Y.; Wang, Y.; Caruso, F. Templated Assembly of pH-Labile Polymer-Drug Particles for Intracellular Drug Delivery. Adv. Funct. Mater. 2012, 22, 4718–4723. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Zhong, Y.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Acetal-linked paclitaxel prodrug micellar nanoparticles as a versatile and potent platform for cancer therapy. Biomacromolecules 2013, 14, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hong, M.; Tang, G.; Qian, L.; Lin, J.; Jiang, Y.; Pei, Y. Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: The effects of PEGylation degree and drug conjugation style. Biomaterials 2010, 31, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhuang, W.; Wang, Y.; Luo, R.; Wang, Y. pH-sensitive doxorubicin-conjugated prodrug micelles with charge-conversion for cancer therapy. Acta Biomater. 2018, 70, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, F.; Zhang, S.; Pollack, S.F.; Elsabahy, M.; Fan, J.; Wooley, K.L. Poly(ethylene oxide)-block-polyphosphoester-graft-paclitaxel conjugates with acid-labile linkages as a pH-sensitive and functional nanoscopic platform for paclitaxel delivery. Adv. Health Mater. 2014, 3, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoh, H.; Miura, Y.; Chida, T.; Liu, X.; Mizuno, K.; Fukushima, S.; Morodomi, Y.; Nishiyama, N.; Cabral, H.; Kataoka, K. Nanomedicines Eradicating Cancer Stem-like Cells in Vivo by pH-Triggered Intracellular Cooperative Action of Loaded Drugs. ACS Nano 2016, 10, 5643–5655. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Li, C.; Shen, X. Charge-reversal nanocarriers: An emerging paradigm for smart cancer nanomedicine. J. Control. Release Off. J. Control. Release Soc. 2020, 319, 46–62. [Google Scholar] [CrossRef]
- Van Driessche, A.; Kocere, A.; Everaert, H.; Nuhn, L.; Van Herck, S.; Griffiths, G.; Fenaroli, F.; De Geest, B.G. pH-Sensitive Hydrazone-Linked Doxorubicin Nanogels via Polymeric-Activated Ester Scaffolds: Synthesis, Assembly, and In Vitro and In Vivo Evaluation in Tumor-Bearing Zebrafish. Chem. Mater. 2018, 30, 8587–8596. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [Green Version]
- Buss, J.H.; Begnini, K.R.; Bruinsmann, F.A.; Ceolin, T.; Sonego, M.S.; Pohlmann, A.R.; Guterres, S.S.; Collares, T.; Seixas, F.K. Lapatinib-Loaded Nanocapsules Enhances Antitumoral Effect in Human Bladder Cancer Cell. Front. Oncol. 2019, 9, 203. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Cho, H.J. Mitochondria Targeting and Destabilizing Hyaluronic Acid Derivative-Based Nanoparticles for the Delivery of Lapatinib to Triple-Negative Breast Cancer. Biomacromolecules 2019, 20, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Xu, J.; Wang, C.; He, F.; Yang, D.; Gai, S.; Yang, P.; Lin, J.; Jin, D.; Xing, B. Tumour microenvironment responsive nanoconstructs for cancer theranostic. Nano Today 2019, 26, 16–56. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer 2008, 8, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release Off. J. Control. Release Soc. 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Wang, Y.; Miao, L.; Xu, Z.; Lin, C.M.; Zhang, Y.; Huang, L. Lipid-coated Cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano 2013, 7, 9896–9904. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Miao, L.; Wang, Y.; Huang, L. Unmodified drug used as a material to construct nanoparticles: Delivery of cisplatin for enhanced anti-cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2014, 174, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.H.; Kwon, G.S. Pre-clinical evaluation of a themosensitive gel containing epothilone B and mTOR/Hsp90 targeted agents in an ovarian tumor model. J. Control Release 2017, 268, 176–183. [Google Scholar] [CrossRef]
- Schnell, C.R.; Stauffer, F.; Allegrini, P.R.; O’Reilly, T.; McSheehy, P.M.; Dartois, C.; Stumm, M.; Cozens, R.; Littlewood-Evans, A.; García-Echeverría, C.; et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: Implications for clinical imaging. Cancer Res. 2008, 68, 6598–6607. [Google Scholar] [CrossRef] [Green Version]
- Guba, M.; von Breitenbuch, P.; Steinbauer, M.; Koehl, G.; Flegel, S.; Hornung, M.; Bruns, C.J.; Zuelke, C.; Farkas, S.; Anthuber, M.; et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med. 2002, 8, 128–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, W.Y.; Huang, L. Systemic delivery of gemcitabine triphosphate via LCP nanoparticles for NSCLC and pancreatic cancer therapy. Biomaterials 2013, 34, 3447–3458. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, Y.; Huang, L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J. Control. Release Off. J. Control. Release Soc. 2012, 158, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Diezi, T.A.; Zhao, A.; Kwon, G.S. Mixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agents. J. Control. Release 2007, 122, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Vicent, M.J. Combination therapy: Opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009, 61, 1203–1213. [Google Scholar] [CrossRef]
- Sirova, M.; Strohalm, J.; Subr, V.; Plocova, D.; Rossmann, P.; Mrkvan, T.; Ulbrich, K.; Rihova, B. Treatment with HPMA copolymer-based doxorubicin conjugate containing human immunoglobulin induces long-lasting systemic anti-tumour immunity in mice. Cancer Immunol. Immunother. 2007, 56, 35–47. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Aryal, S.; Hu, C.M.J.; Zhang, L. Combinatorial Drug Conjugation Enables Nanoparticle Dual-Drug Delivery. Small 2010, 6, 1442–1448. [Google Scholar] [CrossRef]
- Aryal, S.; Hu, C.-M.J.; Zhang, L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol. Pharm. 2011, 8, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef]
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A Convergent Synthetic Platform for Single-Nanoparticle Combination Cancer Therapy: Ratiometric Loading and Controlled Release of Cisplatin, Doxorubicin, and Camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.Y.; Rauth, A.M.; Ronaldson, P.T.; Bendayan, R.; Wu, X.Y. In vitro toxicity to breast cancer cells of microsphere-delivered mitomycin C and its combination with doxorubicin. Eur. J. Pharm. Biopharm. 2006, 62, 321–331. [Google Scholar] [CrossRef] [PubMed]
- De Marre, A.; Soyez, H.; Schacht, E.; Shoaibi, M.A.; Seymour, L.W.; Rihova, B. Synthesis and evaluation of macromolecular prodrugs of mitomycin C. J. Control. Release 1995, 36, 87–97. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, D.; Zhao, P.; Liu, L.; Huang, X.; Qi, C.; Liu, Y.; He, H.; Wang, Q.; Liu, Y.; et al. Intracellular Delivery of Mitomycin C with Targeted Polysaccharide Conjugates Against Multidrug Resistance. Ann. Biomed. Eng. 2011, 39, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gu, Y.; Ma, H.; Bai, J.; Liu, L.; Zhao, P.; He, H. Self-assembled nanoparticle drug delivery systems from galactosylated polysaccharide–doxorubicin conjugate loaded doxorubicin. Int. J. Biol. Macromol. 2010, 46, 245–249. [Google Scholar] [CrossRef]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular drug release nanosystems. Mater. Today 2012, 15, 436–442. [Google Scholar] [CrossRef]
- Xu, W.; Thapa, R.; Liu, D.; Nissinen, T.; Granroth, S.; Närvänen, A.; Suvanto, M.; Santos, H.A.; Lehto, V.-P. Smart Porous Silicon Nanoparticles with Polymeric Coatings for Sequential Combination Therapy. Mol. Pharm. 2015, 12, 4038–4047. [Google Scholar] [CrossRef]
- Nam, J.; La, W.-G.; Hwang, S.; Ha, Y.S.; Park, N.; Won, N.; Jung, S.; Bhang, S.H.; Ma, Y.-J.; Cho, Y.-M.; et al. pH-Responsive Assembly of Gold Nanoparticles and “Spatiotemporally Concerted” Drug Release for Synergistic Cancer Therapy. ACS Nano 2013, 7, 3388–3402. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Tan, X.; Li, J.; Wei, Z.; Zhang, D.; Zheng, Y.; Zheng, A.-X.; Zhao, B.; Zeng, Y.; et al. Photoresponsive Nanovehicle for Two Independent Wavelength Light-Triggered Sequential Release of P-gp shRNA and Doxorubicin To Optimize and Enhance Synergistic Therapy of Multidrug-Resistant Cancer. ACS Appl. Mater. Interfaces 2018, 10, 19416–19427. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, H.; Zeng, D.; Li, Y.; Zheng, Y.; Li, F.; Ji, X.; Wang, X.; Chen, F.; et al. Engineering Inorganic Nanoemulsions/Nanoliposomes by Fluoride-Silica Chemistry for Efficient Delivery/Co-Delivery of Hydrophobic Agents. Adv. Funct. Mater. 2012, 22, 1586–1597. [Google Scholar] [CrossRef]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.C.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Hong, H.; Shi, S.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Engineering of Hollow Mesoporous Silica Nanoparticles for Remarkably Enhanced Tumor Active Targeting Efficacy. Sci. Rep. 2014, 4, 5080. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Talekar, M.; Tran, T.-H.; Samanta, A.; Sundaram, R.; Amiji, M. Combinatorial approach in the design of multifunctional polymeric nano-delivery systems for cancer therapy. J. Mater. Chem. B 2014, 2, 8069–8084. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Kim, H.Y.; Oh, J.Y.; Thomas, A.P.; Choi, E.S.; Jeena, M.T.; Joo, S.H.; Ryu, J.-H. Noncovalent Surface Locking of Mesoporous Silica Nanoparticles for Exceptionally High Hydrophobic Drug Loading and Enhanced Colloidal Stability. Biomacromolecules 2015, 16, 2701–2714. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, G.; Li, Y.; Wang, X.; Liu, S. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J. Am. Chem. Soc. 2015, 137, 362–368. [Google Scholar] [CrossRef]
- Lacroix, P.M.; Graham, S.J.; Lovering, E.G. High-performance liquid chromatographic method for the assay of verapamil hydrochloride and related compounds in raw material. J. Pharm. Biomed. Anal. 1991, 9, 817–822. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Wei, Y.; El-Toni, A.M.; Zhang, F.; Zhao, D. Anisotropic growth-induced synthesis of dual-compartment Janus mesoporous silica nanoparticles for bimodal triggered drugs delivery. J. Am. Chem. Soc. 2014, 136, 15086–15092. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Li, Z.; Li, L.; Saint-Cricq, P.; Li, C.; Lin, J.; Wang, C.; Su, Z.; Zink, J.I. Tailored Synthesis of octopus-type janus nanoparticles for synergistic actively-targeted and chemo-photothermal therapy. Angew. Chem. Int. Ed. 2016, 55, 2118–2121. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, D.; Chang, Z.; Lu, M.; Wang, Y.; Yue, J.; Yang, D.; Li, M.; Xu, Q.; Dong, W.-f. Janus gold nanoplatform for synergetic chemoradiotherapy and computed tomography imaging of hepatocellular carcinoma. ACS Nano 2017, 11, 12732–12741. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Grzelczak, M. Growing anisotropic crystals at the nanoscale. Science 2017, 356, 1120–1121. [Google Scholar] [CrossRef]
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus iron oxides@ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett. 2018, 18, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mei, L.; Feng, S.-S. Paclitaxel drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Pan, J.; Sarisozen, C.; Luther, E.; Torchilin, V. Enhanced cytotoxicity of folic acid-targeted liposomes co-loaded with C6 ceramide and doxorubicin: In vitro evaluation on HeLa, A2780-ADR, and H69-AR cells. Mol. Pharm. 2016, 13, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, Y.-S.; Kim, D.-K. Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology 2009, 84, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, B.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Biodegradable micelles with sheddable poly (ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 2009, 30, 6358–6366. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cheng, Y.; Feng, Y.; Jian, H.; Wang, L.; Ma, X.; Li, X.; Zhang, H. Resonance energy transfer-promoted photothermal and photodynamic performance of gold–copper sulfide yolk–shell nanoparticles for chemophototherapy of cancer. Nano Lett. 2018, 18, 886–897. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.; Xu, L.; Sun, C.; Yang, D.; Xu, J.; He, F.; Gai, S.; Yang, P. A novel core–shell structured upconversion nanorod as a multimodal bioimaging and photothermal ablation agent for cancer theranostics. J. Mater. Chem. B 2018, 6, 2597–2607. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D coordination polymer nanofibers for low-temperature photothermal therapy. Adv. Mater. 2017, 29, 1703588. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Ye, M.; Wang, X.; Tang, J.; Guo, Z.; Shen, Y.; Tian, H.; Zhu, W.H. Dual-channel NIR activatable theranostic prodrug for in vivo spatiotemporal tracking thiol-triggered chemotherapy. Chem. Sci. 2016, 7, 4958–4965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Xiang, J.; Zhu, D.; Jiang, L.; Zhou, Z.; Tang, J.; Liu, X.; Huang, Y.; Shen, Y. Fusogenic Reactive Oxygen Species Triggered Charge-Reversal Vector for Effective Gene Delivery. Adv. Mater. 2016, 28, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Jäättelä, M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 2005, 5, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.; Hsiao, Y.L.; Poon, C.K.; Kwan, P.C.; Chao, S.Y.; Chou, S.T.; Yang, C.S. Glutathione concentration in oral cancer tissues. Cancer Lett. 1994, 81, 111–116. [Google Scholar] [CrossRef]
- Khynriam, D.; Prasad, S.B. Changes in endogenous tissue glutathione level in relation to murine ascites tumor growth and the anticancer activity of cisplatin. Braz. J. Med Biol. Res. 2003, 36, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Antunes, F.; Cadenas, E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): Submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic. Biol. Med. 2001, 30, 1008–1018. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- De Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxidative Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef]
- Yin, W.; Ke, W.; Chen, W.; Xi, L.; Zhou, Q.; Mukerabigwi, J.F.; Ge, Z. Integrated block copolymer prodrug nanoparticles for combination of tumor oxidative stress amplification and ROS-responsive drug release. Biomaterials 2019, 195, 63–74. [Google Scholar] [CrossRef]
- Fang, J.; Deng, D.; Nakamura, H.; Akuta, T.; Qin, H.; Iyer, A.K.; Greish, K.; Maeda, H. Oxystress inducing antitumor therapeutics via tumor-targeted delivery of PEG-conjugated D-amino acid oxidase. Int. J. Cancer 2008, 122, 1135–1144. [Google Scholar] [CrossRef]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin C exhibits pro-oxidant properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kwon, B.; Han, E.; Park, M.; Yang, W.; Cho, W.; Yoo, W.; Khang, G.; Lee, D. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat. Commun. 2015, 6, 6907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Chen, H.; Dong, Y.; Luo, X.; Yu, H.; Moore, Z.; Bey, E.A.; Boothman, D.A.; Gao, J. Superparamagnetic iron oxide nanoparticles: Amplifying ROS stress to improve anticancer drug efficacy. Theranostics 2013, 3, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Chang, S.; Lu, W.; Liu, M.; Pang, X. β-Lapachone Induces NAD(P)H:Quinone Oxidoreductase-1- and Oxidative Stress-Dependent Heat Shock Protein 90 Cleavage and Inhibits Tumor Growth and Angiogenesis. J. Pharmacol. Exp. Ther. 2016, 357, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Li, X.; Duan, X.; Li, M.; Niu, P.; Xu, H.; Cai, K.; Yang, H. A pH/ROS Cascade-Responsive Charge-Reversal Nanosystem with Self-Amplified Drug Release for Synergistic Oxidation-Chemotherapy. Adv. Sci. 2019, 6, 1801807. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Choi, B.T.; Cheong, J.; Choi, Y.H. beta-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-kappaB in human colon cancer HCT-116 cells. Anti-Cancer Drugs 2003, 14, 845–850. [Google Scholar] [CrossRef]
- Woo, H.J.; Park, K.Y.; Rhu, C.H.; Lee, W.H.; Choi, B.T.; Kim, G.Y.; Park, Y.M.; Choi, Y.H. Beta-lapachone, a quinone isolated from Tabebuia avellanedae, induces apoptosis in HepG2 hepatoma cell line through induction of Bax and activation of caspase. J. Med. Food 2006, 9, 161–168. [Google Scholar] [CrossRef]
- Planchon, S.M.; Wuerzberger, S.; Frydman, B.; Witiak, D.T.; Hutson, P.; Church, D.R.; Wilding, G.; Boothman, D.A. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: A p53-independent response. Cancer Res. 1995, 55, 3706–3711. [Google Scholar]
- Shiah, S.G.; Chuang, S.E.; Chau, Y.P.; Shen, S.C.; Kuo, M.L. Activation of c-Jun NH2-terminal kinase and subsequent CPP32/Yama during topoisomerase inhibitor beta-lapachone-induced apoptosis through an oxidation-dependent pathway. Cancer Res. 1999, 59, 391–398. [Google Scholar]
- Zhang, L.; Chen, Z.; Yang, K.; Liu, C.; Gao, J.; Qian, F. β-Lapachone and Paclitaxel Combination Micelles with Improved Drug Encapsulation and Therapeutic Synergy as Novel Nanotherapeutics for NQO1-Targeted Cancer Therapy. Mol. Pharm. 2015, 12, 3999–4010. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.C.; Dou, S.; Xiong, M.H.; Sun, T.M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Vallyathan, V.; Castranova, V.; Shi, X. Oxygen/Nitrogen Radicals: Cell Injury and Disease: Cell Injury and Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; Volume 37. [Google Scholar]
- Leja, K.; Lewandowicz, G. Polymer Biodegradation and Biodegradable Polymers—A Review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Cohen, J.L.; Schubert, S.; Wich, P.R.; Cui, L.; Cohen, J.A.; Mynar, J.L.; Fréchet, J.M.J. Acid-Degradable Cationic Dextran Particles for the Delivery of siRNA Therapeutics. Bioconjug. Chem. 2011, 22, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Teng, W.; Jia, F.; Han, H.; Qin, Z.; Jin, Q.; Ji, J. Polyamino acid-based gemcitabine nanocarriers for targeted intracellular drug delivery. Polym. Chem. 2017, 8, 2490–2498. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, Z.; Wei, W.; Li, Z. Preparation and characterization of plla composite scaffolds by ScCO2-induced phase separation. Polym. Compos. 2012, 33, 1667–1671. [Google Scholar] [CrossRef]
- Dånmark, S.; Finne-Wistrand, A.; Schander, K.; Hakkarainen, M.; Arvidson, K.; Mustafa, K.; Albertsson, A.C. In vitro and in vivo degradation profile of aliphatic polyesters subjected to electron beam sterilization. Acta Biomater. 2011, 7, 2035–2046. [Google Scholar] [CrossRef]
- Sui, G.; Yang, X.; Mei, F.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2007, 82A, 445–454. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostolska, I.; Wiśniewska, M.; Nosal-Wiercińska, A.; Szabelska, A.; Gołębiowska, B. Adsorption layer structure in the system of the ionic block polyamino acid copolymers/SiO2 particles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 138–144. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Binko, A.M.; Traylor, Z.P.; Das, L.M.; Lu, K.Q. 1447 Vitamin D attenuates acute skin inflammation following nitrogen mustard exposure by targeting M1 macrophages. J. Investig. Dermatol. 2018, 138, S245. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, H.; Lu, C.; Yang, C.; Yu, X.; Li, K.; Xie, Y. Novel mitochondria-targeted, nitrogen mustard-based DNA alkylation agents with near infrared fluorescence emission. Talanta 2016, 161, 888–893. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Ye, F.; Dan, G.; Zhao, Y.; Zhao, J.; Zou, Z. Formation and degradation of nitrogen mustard-induced MGMT-DNA crosslinking in 16HBE cells. Toxicology 2017, 389, 67–73. [Google Scholar] [CrossRef]
- Castaño, A.; Roy, U.; Schärer, O.D. Preparation of Stable Nitrogen Mustard DNA Interstrand Cross-Link Analogs for Biochemical and Cell Biological Studies. Methods Enzymol. 2017, 591, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Yan, V.C.; Butterfield, H.E.; Poral, A.H.; Yan, M.J.; Yang, K.L.; Pham, C.-D.; Muller, F.L. Why Great Mitotic Inhibitors Make Poor Cancer Drugs. Trends Cancer 2020, 6, 924–941. [Google Scholar] [CrossRef]
- Blanco, E.; Bey, E.A.; Dong, Y.; Weinberg, B.D.; Sutton, D.M.; Boothman, D.A.; Gao, J. Beta-lapachone-containing PEG-PLA polymer micelles as novel nanotherapeutics against NQO1-overexpressing tumor cells. J. Control. Release Off. J. Control. Release Soc. 2007, 122, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Bey, E.A.; Khemtong, C.; Yang, S.G.; Setti-Guthi, J.; Chen, H.; Kessinger, C.W.; Carnevale, K.A.; Bornmann, W.G.; Boothman, D.A.; et al. Beta-lapachone micellar nanotherapeutics for non-small cell lung cancer therapy. Cancer Res. 2010, 70, 3896–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaucher, G.; Marchessault, R.H.; Leroux, J.C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J. Control. Release Off. J. Control. Release Soc. 2010, 143, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef]
- Eder, K.; Kalman, B. The Dynamics of Interactions Among Immune and Glioblastoma Cells. Neuromol. Med. 2015, 17, 335–352. [Google Scholar] [CrossRef]
- Waghray, M.; Yalamanchili, M.; di Magliano, M.P.; Simeone, D.M. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 2013, 29, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Criscitiello, C.; Esposito, A.; Curigliano, G. Tumor-stroma crosstalk: Targeting stroma in breast cancer. Curr. Opin. Oncol. 2014, 26, 551–555. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.; Ahn, M.; Jang, H.; Min, D.H. Development of Dual-Pore Coexisting Branched Silica Nanoparticles for Efficient Gene-Chemo Cancer Therapy. Small 2018, 14, 1702564. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010, 21, 21–26. [Google Scholar] [CrossRef]
- Kelly, M.G.; Alvero, A.B.; Chen, R.; Silasi, D.A.; Abrahams, V.M.; Chan, S.; Visintin, I.; Rutherford, T.; Mor, G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006, 66, 3859–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Alvero, A.B.; Silasi, D.A.; Mor, G. Inflammation, cancer and chemoresistance: Taking advantage of the toll-like receptor signaling pathway. Am. J. Reprod. Immunol. 2007, 57, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Zhao, Z. Discovery and design of selective cyclooxygenase-2 inhibitors as non-ulcerogenic, anti-inflammatory drugs with potential utility as anti-cancer agents. Curr. Drug Targets 2001, 2, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Shao, J.; Morrow, J.D.; Beauchamp, R.D.; DuBois, R.N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998, 58, 362–366. [Google Scholar] [PubMed]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelman, M.J.; Wang, X.; Hodgson, L.; Cheney, R.T.; Baggstrom, M.Q.; Thomas, S.P.; Gajra, A.; Bertino, E.; Reckamp, K.L.; Molina, J.; et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; McMillan, D.C.; Horgan, P.G.; Roxburgh, C.S. The impact of anti-inflammatory agents on the outcome of patients with colorectal cancer. Cancer Treat. Rev. 2014, 40, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takhar, H.; Singhal, N.; Mislang, A.; Kumar, R.; Kim, L.; Selva-Nayagam, S.; Pittman, K.; Karapetis, C.; Borg, M.; Olver, I.N.; et al. Phase II study of celecoxib with docetaxel chemoradiotherapy followed by consolidation chemotherapy docetaxel plus cisplatin with maintenance celecoxib in inoperable stage III nonsmall cell lung cancer. Asia-Pac. J. Clin. Oncol. 2018, 14, 91–100. [Google Scholar] [CrossRef]
- Mohammed, A.; Yarla, N.S.; Madka, V.; Rao, C.V. Clinically Relevant Anti-Inflammatory Agents for Chemoprevention of Colorectal Cancer: New Perspectives. Int. J. Mol. Sci. 2018, 19, 2332. [Google Scholar] [CrossRef] [Green Version]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar]
- Nagase, H.; Fields, G.B. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers 1996, 40, 399–416. [Google Scholar] [CrossRef]
- Dorresteijn, R.; Billecke, N.; Schwendy, M.; Pütz, S.; Bonn, M.; Parekh, S.H.; Klapper, M.; Müllen, K. Polylactide-block-Polypeptide-block-Polylactide Copolymer Nanoparticles with Tunable Cleavage and Controlled Drug Release. Adv. Funct. Mater. 2014, 24, 4026–4033. [Google Scholar] [CrossRef]
- Shuai, X.; Merdan, T.; Unger, F.; Wittmar, M.; Kissel, T. Novel Biodegradable Ternary Copolymers hy-PEI-g-PCL-b-PEG: Synthesis, Characterization, and Potential as Efficient Nonviral Gene Delivery Vectors. Macromolecules 2003, 36, 5751–5759. [Google Scholar] [CrossRef]
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J. Control. Release Off. J. Control. Release Soc. 2004, 98, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Sunitinib: A VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem. Biophys. Res. Commun. 2007, 356, 323–328. [Google Scholar] [CrossRef]
- Bergh, J.; Bondarenko, I.M.; Lichinitser, M.R.; Liljegren, A.; Greil, R.; Voytko, N.L.; Makhson, A.N.; Cortes, J.; Lortholary, A.; Bischoff, J.; et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: Results of a prospective, randomized phase III study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 921–929. [Google Scholar] [CrossRef]
- Camidge, D.R.; Blais, N.; Jonker, D.J.; Soulières, D.; Doebele, R.C.; Ruiz-Garcia, A.; Thall, A.; Zhang, K.; Laurie, S.A.; Chao, R.C.; et al. Sunitinib combined with pemetrexed and cisplatin: Results of a phase I dose-escalation and pharmacokinetic study in patients with advanced solid malignancies, with an expanded cohort in non-small cell lung cancer and mesothelioma. Cancer Chemother. Pharmacol. 2013, 71, 307–319. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Powles, T.; Hellerstedt, B.A.; Lerner, S.P.; Gardner, T.A.; Yu, M.; O’Rourke, M.; Vogelzang, N.J.; Kocs, D.; et al. Gemcitabine, Cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin. Genitourin. Cancer 2013, 11, 175–181. [Google Scholar] [CrossRef]
- Christensen, J.G. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18 Suppl 10, x3–10. [Google Scholar] [CrossRef]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. 2003, 115, 4788–4791. [Google Scholar] [CrossRef]
- Rihova, B.; Etrych, T.; Sirova, M.; Kovar, L.; Hovorka, O.; Kovar, M.; Benda, A.; Ulbrich, K. Synergistic action of doxorubicin bound to the polymeric carrier based on N-(2-hydroxypropyl) methacrylamide copolymers through an amide or hydrazone bond. Mol. Pharm. 2010, 7, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kim, D. pH-Sensitive micelles with cross-linked cores formed from polyaspartamide derivatives for drug delivery. Langmuir 2011, 27, 12090–12097. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xiao, J.; Yin, Q.; Zhang, Z.; Yu, H.; Mao, S.; Li, Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano 2013, 7, 5858–5869. [Google Scholar] [CrossRef] [PubMed]








| Release Type | Year | Nano Carrier | Used Drug | Research Progress | Author |
|---|---|---|---|---|---|
| Ratiometric drug release | 2018 | VES-g-ε-PLL, dopamine-modified-poly-γ-glutamic acid polymer (γ-PGA-Dopa) | Doxurbicin (DOX), Curcumin | In vitro/in vivo | Xu et al. [28] |
| 2020 | PEGylated ε-poly-l-lysine polymeric nanoparticles | DOX, Lapatinib | In vitro/in vivo | Guo et al. [29] | |
| 2014 | Dioleoyl phosphatidic acid, PLGA-PEG-Anisamide NPs | Cisplatin, Gemcitabine monophosphate | In vitro/in vivo | Miao et al. [30] | |
| 2014 | Poly(lactic-co-glycolic acid) (PLGA) NPs | Rapamycin, Cisplatin | In vitro/in vivo | Guo et al. [31] | |
| 2015 | Xyloglucan, tripeptide Gly-Leu-Gly | DOX, Mitomycin C | In vitro/in vivo | Luo et al. [32] | |
| Sequential drug release in intracellular | 2017 | Hollow mesoporous silica nanoparticles (HMSNs), PEG-PDS-DPA copolymer | Verapamil∙HCl, DOX | In vitro | Palanikumar et al. [33] |
| 2018 | Janus nanoparticles | DOX, Docetaxel | In vitro/in vivo | Zhang et al. [34] | |
| 2017 | Poly(ethyleneglycol)-poly [2-(methylacryloyl)ethylnicotinate] (PEG-PMAN) | β-Lapachone, ROS-responsive doxorubicin (DOX) prodrug | In vitro/in vivo | Ye et al. [35] | |
| 2019 | mPEG-acetalated maltoheptaose (AcMH)Poly(aspartic acid)(PAsp)-AcMH | β-Lapachone, Niatrogen mustard (NM) prodrug | In vitro/in vivo | Luan et al. [36] | |
| 2019 | PEG-b-poly(d,l-lactic acid) (PDLLA) | β-Lapachone, Oxidation-resposive thioether-linked linoleic aicd-paclitaxel conjugates (PTX-S-LA) | In vitro/in vivo | Wang et al. [37] | |
| Spatiotemporal sequential drug release | 2019 | [PPLG-g-(CXB-peptide & mPEG)]-PEG-PCL (PCxbP) | Paclitaxel, Celecoxib | In vitro/in vivo | Huang et al. [38] |
| 2019 | mPEG-PLLMA(peptide-CD)-PAsp(DBP) | Paclitaxel, Sunitinib | In vitro/in vivo | He et al. [39] | |
| 2015 | poly(ethylene glycol)-polyhistidine (PEG-Phis) polypeptide | Doxorubicin, Combretastatin A4 | In vitro/in vivo | Dong et al. [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, M.S.; Lee, Y.J.; Shin, H.J.; Park, C.-W.; Han, S.-B.; Jung, J.-K.; Kim, J.-S.; Shin, D.H. Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles. Pharmaceutics 2020, 12, 1156. https://doi.org/10.3390/pharmaceutics12121156
Yoon MS, Lee YJ, Shin HJ, Park C-W, Han S-B, Jung J-K, Kim J-S, Shin DH. Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles. Pharmaceutics. 2020; 12(12):1156. https://doi.org/10.3390/pharmaceutics12121156
Chicago/Turabian StyleYoon, Moon Sup, Yu Jin Lee, Hee Ji Shin, Chun-Woong Park, Sang-Bae Han, Jae-Kyung Jung, Jin-Seok Kim, and Dae Hwan Shin. 2020. "Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles" Pharmaceutics 12, no. 12: 1156. https://doi.org/10.3390/pharmaceutics12121156