Blood–Brain Barrier Modulation to Improve Glioma Drug Delivery
Abstract
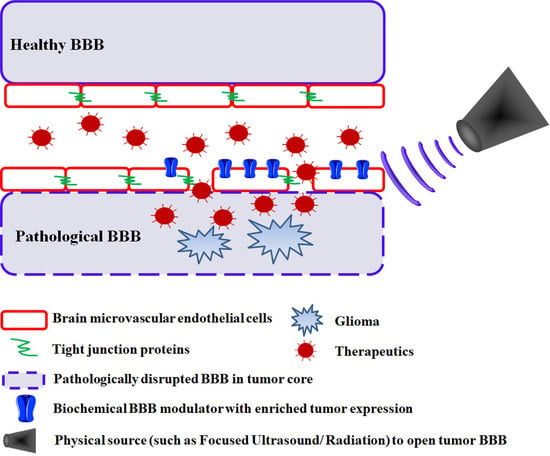
:1. Introduction
2. Leveraging Pathological BBB Disruption in Glioma
2.1. Passive Drug Accumulation at Sites of Tumor BBB Disruption
2.2. Targeted Drug Delivery at Sites of Tumor BBB Disruption
3. Biochemical Modulation
3.1. ATP-Sensitive Potassium Channel Activators
3.2. Calcium-Activated Potassium Channel Activators
3.3. Phosphodiesterase 5 (PDE5) Inhibitors
3.4. Bradykinin Type 2 Receptor Activators
3.5. Adenosine 2A Receptor Activators
3.6. Papaverine
3.7. microRNAs
4. Physical Modulation
4.1. Electromagnetic Pulse (EMP)
4.2. Laser-Induced Thermal Therapy (LITT)
4.3. Radiotherapy: Synchrotron Microbeam Radiation Therapy (MRT)
4.4. Focused Ultrasound (FUS)
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, A.L.; Colman, H. Glioma biology and molecular markers. Cancer Treat. Res. 2015, 163, 15–30. [Google Scholar] [PubMed]
- Mrugala, M.M. Advances and challenges in the treatment of glioblastoma: A clinician’s perspective. Discov. Med. 2013, 15, 221–230. [Google Scholar] [PubMed]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16, iv1–iv63. [Google Scholar] [CrossRef] [PubMed]
- Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Improving drug delivery to primary and metastatic brain tumors: Strategies to overcome the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 336–346. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood–brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Sundgren, P.C.; Tsien, C.I.; Chenevert, T.T.; Junck, L. Physiologic and Metabolic Magnetic Resonance Imaging in Gliomas. J. Clin. Oncol. 2006, 24, 1228–1235. [Google Scholar] [CrossRef]
- Prabhu, S.S.; Broaddus, W.C.; Oveissi, C.; Berr, S.S.; Gillies, G.T. Determination of intracranial tumor volumes in a rodent brain using magnetic resonance imaging, evans blue, and histology: A comparative study. IEEE Trans. Bio-Med. Eng. 2000, 47, 259–265. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma. Neuro-Oncology 2015, 18, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Kim, D.W.; Lee, S.-K.; Chang, J.H.; Kang, S.-G.; Kim, E.-H.; Kim, S.H.; Rim, T.H.; Ahn, S.S. The Added Prognostic Value of Preoperative Dynamic Contrast-Enhanced MRI Histogram Analysis in Patients with Glioblastoma: Analysis of Overall and Progression-Free Survival. Am. J. Neuroradiol. 2015, 36, 2235–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arevaloperez, J.; Thomas, A.; Kaley, T.J.; Lyo, J.K.; Peck, K.K.; Holodny, A.; Mellinghoff, I.K.; Shi, W.; Zhang, Z.; Young, R. T1-Weighted Dynamic Contrast-Enhanced MRI as a Noninvasive Biomarker of Epidermal Growth Factor Receptor vIII Status. Am. J. Neuroradiol. 2015, 36, 2256–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarosa, C.; Castellano, A.; Conte, G.M.; Cadioli, M.; Iadanza, A.; Terreni, M.R.; Franzin, A.; Bello, L.; Caulo, M.; Falini, A.; et al. Dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging for glioma grading: Preliminary comparison of vessel compartment and permeability parameters using hotspot and histogram analysis. Eur. J. Radiol. 2016, 85, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Yang, S.; Babb, J.S.; A Knopp, E.; Golfinos, J.G.; Zagzag, D.; Johnson, G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. Am. J. Neuroradiol. 2004, 25, 746–755. [Google Scholar] [PubMed]
- Onda, K.; Tanaka, R.; Takahashi, H.; Takeda, N.; Ikuta, F. Cerebral Glioblastoma with Cerebrospinal Fluid Dissemination: A Clinicopathological Study of 14 Cases Examined by Complete Autopsy. Neurosurg. 1989, 25, 533–540. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Alipour, M.; Fricker, G.; Miller, D.S.; Kugler, W.; Eibl, H.; Lakomek, M. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br. J. Pharmacol. 2003, 140, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Abdul Razzak, R.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS Drug Delivery with a Focus on Transporter-Mediated Transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling Drugs into the Brain: An Overview of Ligands Targeting Transcytosis for Drug Delivery across the Blood–Brain Barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef] [Green Version]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-Promoting Effect of Blood? Brain Barrier Disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yue, Q.; Liu, Y.; Fan, D.; Fan, K.; Li, S.; Qian, J.; Han, L.; Fang, F.; Xu, F.; et al. Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins. Theranostics 2018, 8, 3126–3137. [Google Scholar] [CrossRef]
- Ichimura, K.; Ohno, K.; Aoyagi, M.; Tamaki, M.; Suzuki, R.; Hirakawa, K. Capillary permeability in experimental rat glioma and effects of intracarotid CDDP administration on tumor drug delivery. J. Neuro-Oncol. 1993, 16, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Jain, R. Measurements of tumor vascular leakiness using DCE in brain tumors: Clinical applications. NMR Biomed. 2013, 26, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Giovannelli, G.; Fiaschini, P.; Muti, M.; Kouleridou, A.; Caputo, N. High- and low-grade glioma differentiation: The role of percentage signal recovery evaluation in MR dynamic susceptibility contrast imaging. La Radiol. Medica 2015, 120, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Gerlowski, L.E.; Jain, R.K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Nugent, L.J.; Jain, R.K. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984, 44, 238–244. [Google Scholar]
- Provenzale, J.M.; Mukundan, S.; Dewhirst, M. The Role of Blood-Brain Barrier Permeability in Brain Tumor Imaging and Therapeutics. Am. J. Roentgenol. 2005, 185, 763–767. [Google Scholar] [CrossRef]
- Umlauf, B.J.; Shusta, E.V. Exploiting BBB disruption for the delivery of nanocarriers to the diseased CNS. Curr. Opin. Biotechnol. 2019, 60, 146–152. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Adkins, C.E.; Bohn, K.A.; Mohammad, A.S.; Lockman, J.A.; Lockman, P.R. Quantitative Fluorescence Microscopy Measures Vascular Pore Size in Primary and Metastatic Brain Tumors. Cancer Res. 2016, 77, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; A Aronova, M.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Goldwirt, L.; Beccaria, K.; Carpentier, A.; Farinotti, R.; Fernandez, C. Irinotecan and temozolomide brain distribution: A focus on ABCB1. Cancer Chemother. Pharmacol. 2014, 74, 185–193. [Google Scholar] [CrossRef]
- De Vries, N.A.; Zhao, J.; Kroon, E.; Buckle, T.; Beijnen, J.H.; Van Tellingen, O. P-Glycoprotein and Breast Cancer Resistance Protein: Two Dominant Transporters Working Together in Limiting the Brain Penetration of Topotecan. Clin. Cancer Res. 2007, 13, 6440–6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dréan, A.; Goldwirt, L.; Verreault, M.; Canney, M.; Schmitt, C.; Guehennec, J.; Delattre, J.-Y.; Carpentier, A.; Idbaih, A. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev. Neurother. 2016, 16, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S. Selective drug delivery approaches to lesioned brain through blood brain barrier disruption. Expert Opin. Drug Deliv. 2018, 15, 335–349. [Google Scholar] [CrossRef]
- Front, D.; Israel, O.; Kohn, S.; Nir, I. The blood-tissue barrier of human brain tumors: Correlation of scintigraphic and ultrastructural findings: Concise communication. J. Nucl. Med. 1984, 25, 461–465. [Google Scholar]
- Siegal, T.; Horowitz, A.; Gabizon, A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: Biodistribution and therapeutic efficacy. J. Neurosurg. 1995, 83, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Krauze, M.T.; Noble, C.O.; Kawaguchi, T.; Drummond, D.; Kirpotin, D.B.; Yamashita, Y.; Kullberg, E.; Forsayeth, J.; Park, J.W.; Bankiewicz, K.S. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro-Oncology 2007, 9, 393–403. [Google Scholar] [CrossRef]
- Fabel, K.; Dietrich, J.; Hau, P.; Wismeth, C.; Winner, B.; Przywara, S.; Steinbrecher, A.; Ullrich, W.; Bogdahn, U. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 2001, 92, 1936–1942. [Google Scholar] [CrossRef]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C.; et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer 2004, 100, 1199–1207. [Google Scholar] [CrossRef]
- Chua, S.L.; A Rosenthal, M.; Wong, S.S.; Ashley, D.M.; Woods, A.-M.; Dowling, A.J.; Cher, L.M. Phase 2 study of temozolomide and Caelyx in patients with recurrent glioblastoma multiforme. Neuro-Oncology 2004, 6, 38–43. [Google Scholar] [CrossRef]
- Glas, M.; Koch, H.; Hirschmann, B.; Jauch, T.; Steinbrecher, A.; Herrlinger, U.; Bogdahn, U.; Hau, P. Pegylated Liposomal Doxorubicin in Recurrent Malignant Glioma: Analysis of a Case Series. Oncology 2007, 72, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma - a phase II study. BMC Cancer 2009, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umlauf, B.J.; A Clark, P.; Lajoie, J.M.; Georgieva, J.V.; Bremner, S.; Herrin, B.R.; Kuo, J.S.; Shusta, E.V. Identification of variable lymphocyte receptors that can target therapeutics to pathologically exposed brain extracellular matrix. Sci. Adv. 2019, 5, eaau4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Yan, X.; Liu, Y.; Huang, L.; Kong, C.; Qu, X.; Wang, M.; Gao, R.; Qin, H.-L. Application of dual targeting drug delivery system for the improvement of anti-glioma efficacy of doxorubicin. Oncotarget 2017, 8, 58823–58834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarboe, J.S.; Johnson, K.R.; Choi, Y.; Lonser, R.R.; Park, J.K. Expression of Interleukin-13 Receptor α2 in Glioblastoma Multiforme: Implications for Targeted Therapies. Cancer Res. 2007, 67, 7983–7986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debinski, W.; Gibo, D.M.; Slagle, B.; Powers, S.K.; Gillespie, G.Y. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int. J. Oncol. 1999, 15. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.; Slagle-Webb, B.; Mintz, A.; Sheehan, J.M.; Connor, J.R. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol. Cancer Ther. 2006, 5, 3162–3169. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Coveñas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, S.; Yang, Z.; Cao, S.; Jiang, X.; Pang, Z. In vitro and in vivo intracellular distribution and anti-glioblastoma effects of docetaxel-loaded nanoparticles functioned with IL-13 peptide. Int. J. Pharm. 2014, 466, 8–17. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Shen, S.; Pang, Z.; Jiang, X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci. Rep. 2013, 3, srep02534. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Pang, Z.; Yang, X.; Jiang, X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J. Control. Release 2013, 172, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Mintz, A.; Debinski, W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia 2004, 6, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wu, G.; Wang, H.; Xu, B. Pep-1&borneol–Bifunctionalized Carmustine-Loaded Micelles Enhance Anti-Glioma Efficacy through Tumor-Targeting and BBB-Penetrating. J. Pharm. Sci. 2019, 108, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lv, L.; Shi, H.; Hua, Y.; Lv, W.; Wang, X.; Xin, H.; Xu, Q. PEGylated Polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma. Colloids Surf. B Biointerfaces 2016, 147, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Sun, T.; et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, X.; Wang, Q.; Chen, Q.; Lu, Y.; Liu, L.; Zhang, Y.; He, X.; Ruan, C.; Zhang, Y.; et al. Substance P Mediated DGLs Complexing with DACHPt for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2017, 9, 34603–34617. [Google Scholar] [CrossRef] [PubMed]
- Ningaraj, N.S.; Rao, M.K.; Black, K.L. Adenosine 5’-triphosphate-sensitive potassium channel-mediated blood-brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003, 63, 8899–8911. [Google Scholar]
- Gu, Y.-T.; Xue, Y.-X.; Zhang, H.; Li, Y.; Liang, X.-Y. Adenosine 5′-Triphosphate-Sensitive Potassium Channel Activator Induces the Up-Regulation of Caveolin-1 Expression in a Rat Brain Tumor Model. Cell. Mol. Neurobiol. 2011, 31, 629–634. [Google Scholar] [CrossRef]
- Gu, Y.-T.; Xue, Y.; Wang, Y.; Wang, J.-H.; Chen, X.; Shangguan, Q.-R.; Lian, Y.; Zhong, L.; Meng, Y.-N. Minoxidil sulfate induced the increase in blood–brain tumor barrier permeability through ROS/RhoA/PI3K/PKB signaling pathway. Neuropharmacology 2013, 75, 407–415. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.; Hashizume, K.; Asotra, K.; Black, K.L. Regulation of Blood-Brain Tumor Barrier Permeability by Calcium-Activated Potassium Channels. J. Pharmacol. Exp. Ther. 2002, 301, 838–851. [Google Scholar] [CrossRef] [Green Version]
- Ningaraj, N.S.; Rao, M.; Black, K.L. Calcium-dependent potassium channels as a target protein for modulation of the blood-brain tumor barrier. Drug News Perspect. 2003, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yuan, X.; Ko, M.K.; Yin, D.; Sacapano, M.R.; Wang, X.; Konda, B.; Espinoza, A.; Prosolovich, K.; Ong, J.M.; et al. Calcium-activated potassium channels mediated blood-brain tumor barrier opening in a rat metastatic brain tumor model. Mol. Cancer 2007, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ningaraj, N.; Khaitan, D. Evidence of calcium-activated potassium channel subunit alpha-1 as a key promoter of glioma growth and tumorigenicity. Glioma 2019, 2, 46. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Sankpal, U.T.; Khaitan, D.; Meister, E.A.; Vats, T.S. Modulation of KCa channels increases anticancer drug delivery to brain tumors and prolongs survival in xenograft model. Cancer Biol. Ther. 2009, 8, 1924–1933. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.-P.; Xue, Y.-X.; Huang, J.; Wang, J.-H.; Wang, J.-H.; Zhao, S.-Y.; Guan, T.-T.; Zhang, Z.; Gu, Y.-T. NS1619 regulates the expression of caveolin-1 protein in a time-dependent manner via ROS/PI3K/PKB/FoxO1 signaling pathway in brain tumor microvascular endothelial cells. J. Neurol. Sci. 2016, 369, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Michel, C. Capillaries, Caveolae, Calcium and Cyclic Nucleotides: A New Look at Microvascular Permeability. J. Mol. Cell. Cardiol. 1998, 30, 2541–2546. [Google Scholar] [CrossRef]
- Juilfs, D.M.; Soderling, S.; Burns, F.; Beavo, J.A. Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs). Rev. Physiol. Biochem. Pharmacol. 1999, 135, 67–104. [Google Scholar] [CrossRef]
- Black, K.L.; Yin, D.; Ong, J.M.; Hu, J.; Konda, B.M.; Wang, X.; Ko, M.K.; Bayan, J.A.; Sacapano, M.R.; Espinoza, A.; et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008, 1230, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Cesarini, V.; Martini, M.; Vitiani, L.R.; Gravina, G.L.; Di Agostino, S.; Graziani, G.; D’Alessandris, Q.G.; Pallini, R.; LaRocca, L.M.; Rossi, P.; et al. Type 5 phosphodiesterase regulates glioblastoma multiforme aggressiveness and clinical outcome. Oncotarget 2017, 8, 13223–13239. [Google Scholar] [CrossRef]
- Hu, J.; Ljubimova, J.Y.; Inoue, S.; Konda, B.; Patil, R.; Ding, H.; Espinoza, A.; Wawrowsky, K.A.; Patil, C.; Ljubimov, A.V.; et al. Phosphodiesterase Type 5 Inhibitors Increase Herceptin Transport and Treatment Efficacy in Mouse Metastatic Brain Tumor Models. PLoS ONE 2010, 5, e10108. [Google Scholar] [CrossRef] [Green Version]
- Inamura, T.; Black, K.L. Bradykinin Selectively Opens Blood-Tumor Barrier in Experimental Brain Tumors. Br. J. Pharmacol. 1994, 14, 862–870. [Google Scholar] [CrossRef] [Green Version]
- Raymond, J.J.; Robertson, D.M.; Dinsdale, H.B. Pharmacological Modification of Bradykinin Induced Breakdown of the Blood-brain Barrier. Can. J. Neurol. Sci. 1986, 13, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerich, D.F.; Dean, R.L.; Osborn, C.; Bartus, R.T. The Development of the Bradykinin Agonist Labradimil as a Means to Increase the Permeability of the Blood-Brain Barrier. Clin. Pharmacokinet. 2001, 40, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Q.; Xie, C.; Lu, W.; Peng, C.; Wei, X.; Li, X.; Su, B.; Gao, C.; Liu, M. Retro-inverso bradykinin opens the door of blood–brain tumor barrier for nanocarriers in glioma treatment. Cancer Lett. 2015, 369, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, B.; Wang, R.; Xie, Z.; Ruan, H.; Li, J.; Xie, C.; Lu, W.; Wang, J.; Wang, D.; Liu, M. Effect of Retro-Inverso Isomer of Bradykinin on Size-Dependent Penetration of Blood-Brain Tumor Barrier. Small 2018, 14. [Google Scholar] [CrossRef]
- Bartus, R.; Elliott, P.; Hayward, N.; Dean, R.; McEwen, E.; Fisher, S. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: Evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology 1996, 33, 270–278. [Google Scholar] [CrossRef]
- Uchida, M.; Chen, Z.; Liu, Y.; Black, K.L. Overexpression of bradykinin type 2 receptors on glioma cells enhances bradykinin-mediated blood–brain tumor barrier permeability increase. Neurol. Res. 2002, 24, 739–746. [Google Scholar] [CrossRef]
- Liu, Y.; Hashizume, K.; Chen, Z.; Samoto, K.; Ningaraj, N.; Asotra, K.; Black, K.L. Correlation between bradykinin-induced blood–tumor barrier permeability and B2 receptor expression in experimental brain tumors. Neurol. Res. 2001, 23, 379–387. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, Y.; Liu, Y.; Fu, W.; Jiang, N.; An, P.; Wang, P.; Yang, Z.; Wang, Y. Study of correlation between expression of bradykinin B 2 receptor and pathological grade in human gliomas. Br. J. Neurosurg. 2005, 19, 322–326. [Google Scholar] [CrossRef]
- Cote, J.; Savard, M.; Bovenzi, V.; Dubuc, C.; Tremblay, L.; Tsanaclis, A.M.; Fortin, D.; Lepage, M.; Gobeil, F., Jr. Selective tumor blood-brain barrier opening with the kinin B2 receptor agonist [Phe(8)psi(CH(2)NH)Arg(9)]-BK in a F98 glioma rat model: An MRI study. Neuropeptides 2010, 44, 177–185. [Google Scholar] [CrossRef]
- Liu, L.B.; Xue, Y.X.; Liu, Y.H.; Wang, Y.B. Bradykinin increases blood-tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J. Neurosci. Res. 2008, 86, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Carman, A.J.; Mills, J.H.; Krenz, A.; Kim, D.-G.; Bynoe, M.S. Adenosine Receptor Signaling Modulates Permeability of the Blood-Brain Barrier. J. Neurosci. 2011, 31, 13272–13280. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Bynoe, M.S. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Mol. Neurobiol. 2014, 52, 664–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Bai, Y.-Y.; Liu, Y.; Gao, X.; Li, Y.; Changyi, Y.; Wang, Y.; Chang, D.; Ju, S.-H.; Li, C. Salvaging brain ischemia by increasing neuroprotectant uptake via nanoagonist mediated blood brain barrier permeability enhancement. Biomaterials 2015, 66, 9–20. [Google Scholar] [CrossRef]
- Jin, Q.; Cai, Y.; Li, S.; Liu, H.; Zhou, X.; Lu, C.; Gao, X.; Qian, J.; Zhang, J.; Ju, S.; et al. Edaravone-Encapsulated Agonistic Micelles Rescue Ischemic Brain Tissue by Tuning Blood-Brain Barrier Permeability. Theranostics 2017, 7, 884–898. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, A.K.; Kondoh, T.; Nagashima, T.; Ikeda, M.; Ehara, K.; Tamaki, N. Quantitative Analysis of Papaverine-Mediated Blood–Brain Barrier Disruption in Rats. Biochem. Biophys. Res. Commun. 2001, 289, 548–552. [Google Scholar] [CrossRef]
- Platz, J.; Barath, K.; Keller, E.; Valavanis, A. Disruption of the blood-brain barrier by intra-arterial administration of papaverine: A technical note. Neuroradiology 2008, 50, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Wang, H.; Kong, L.; Zhou, H. Opening blood-brain-barrier by intracarotid infusion of papaverine in treatment of malignant cerebral glioma. Chin. Med. J. 1998, 111, 751–753. [Google Scholar]
- Wang, Z.-H.; Xue, Y.; Liu, Y.-H. The modulation of protein kinase A and heat shock protein 70 is involved in the reversible increase of blood–brain tumor barrier permeability induced by papaverine. Brain Res. Bull. 2010, 83, 367–373. [Google Scholar] [CrossRef]
- Gu, Y.; Cai, R.; Zhang, C.; Xue, Y.; Pan, Y.; Wang, J.; Zhang, Z. miR-132-3p boosts caveolae-mediated transcellular transport in glioma endothelial cells by targeting PTEN/PI3K/PKB/Src/Cav-1 signaling pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 441–454. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Peng, C.; Liu, Y.-H. Low dose of bradykinin selectively increases intracellular calcium in glioma cells. J. Neurol. Sci. 2007, 258, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.B.; Xue, Y.X.; Liu, Y.H. Bradykinin increases the permeability of the blood-tumor barrier by the caveolae-mediated transcellular pathway. J. Neuro-Oncol. 2010, 99, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, Y.T.; Xue, Y.X. Bradykinin-induced blood-brain tumor barrier permeability increase is mediated by adenosine 5’-triphosphate-sensitive potassium channel. Brain Res. 2007, 1144, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M. Bradykinin receptors. Gen. Pharmacol. 1997, 28, 1–6. [Google Scholar] [CrossRef]
- Venema, R.C. Post-translational mechanisms of endothelial nitric oxide synthase regulation by bradykinin. Int. Immunopharmacol. 2002, 2, 1755–1762. [Google Scholar] [CrossRef]
- Nakano, S.; Matsukado, K.; Black, K.L. Increased brain tumor microvessel permeability after intracarotid bradykinin infusion is mediated by nitric oxide. Cancer Res. 1996, 56, 4027–4031. [Google Scholar]
- Sugita, M.; Black, K.L. Cyclic GMP-specific phosphodiesterase inhibition and intracarotid bradykinin infusion enhances permeability into brain tumors. Cancer Res. 1998, 58, 914–920. [Google Scholar]
- Khan, F.; Pearson, R.J.; Newton, D.J.; Belch, J.J.; Butler, A.R. Chemical synthesis and microvascular effects of new nitric oxide donors in humans. Clin. Sci. 2003, 105, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hashizume, K.; Samoto, K.; Sugita, M.; Ningaraj, N.; Asotra, K.; Black, K.L. Repeated, short-term ischemia augments bradykinin-mediated opening of the blood–tumor barrier in rats with RG2 glioma. Neurol. Res. 2001, 23, 631–640. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Konda, B.; Ong, J.M.; Hu, J.; Sacapano, M.R.; Ko, M.K.; Espinoza, A.J.; Irvin, D.K.; Shu, Y.; et al. Increase in Brain Tumor Permeability in Glioma-Bearing Rats with Nitric Oxide Donors. Clin. Cancer Res. 2008, 14, 4002–4009. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.; Weingart, J.; Nduom, E.K.; Harfi, T.T.; George, R.T.; McAreavey, D.; Ye, X.; Anders, N.M.; Peer, C.; Figg, W.D.; et al. The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.; George, R.T.; Lodge, M.A.; Piotrowski, A.; Wahl, R.L.; Gujar, S.K.; Grossman, S. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J. Neuro-Oncol. 2017, 132, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Sırav, B.; Seyhan, N. Effects of GSM modulated radio-frequency electromagnetic radiation on permeability of blood–brain barrier in male & female rats. J. Chem. Neuroanat. 2016, 75, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Salford, L.G.; Brun, A.; Sturesson, K.; Eberhardt, J.L.; Persson, B. Permeability of the blood-brain barrier induced by 915 MHz electromagnetic radiation, continuous wave and modulated at 8, 16, 50, and 200 Hz. Microsc. Res. Tech. 1994, 27, 535–542. [Google Scholar] [CrossRef]
- Zhou, J.X.; Ding, G.R.; Zhang, J.; Zhou, Y.C.; Zhang, Y.J.; Guo, G.Z. Detrimental effect of electromagnetic pulse exposure on permeability of in vitro blood-brain-barrier model. Biomed. Environ. Sci. 2013, 26, 128–137. [Google Scholar] [PubMed]
- Ding, G.-R.; Li, K.-C.; Wang, X.-W.; Zhou, Y.-C.; Qiu, L.-B.; Tan, J.; Xu, S.-L.; Guo, G.-Z. Effect of Electromagnetic Pulse Exposure on Brain Micro Vascular Permeability in Rats. Biomed. Environ. Sci. 2009, 22, 265–268. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhou, Y.; Qiu, L.B.; Ding, G.R.; Pang, X.F. Altered expression of matrix metalloproteinases and tight junction proteins in rats following PEMF-induced BBB permeability change. Biomed. Environ. Sci. 2012, 25, 197–202. [Google Scholar]
- Li, K.; Zhang, K.; Xu, S.; Wang, X.; Zhou, Y.; Zhou, Y.; Gao, P.; Lin, J.; Ding, G.; Guo, G.-Z. EMP-induced BBB-disruption enhances drug delivery to glioma and increases treatment efficacy in rats. Bioelectromagnetics 2017, 39, 60–67. [Google Scholar] [CrossRef]
- Carpentier, A.; Chauvet, D.; Reina, V.; Beccaria, K.; LeClerq, D.; McNichols, R.J.; Gowda, A.; Cornu, P.; Delattre, J.-Y. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg. Med. 2012, 44, 361–368. [Google Scholar] [CrossRef]
- Sabel, M.; Rommel, F.; Kondakci, M.; Gorol, M.; Willers, R.; Bilzer, T. Locoregional opening of the rodent blood-brain barrier for paclitaxel using Nd:YAG laser-induced thermo therapy: A new concept of adjuvant glioma therapy? Lasers Surg. Med. 2003, 33, 75–80. [Google Scholar] [CrossRef]
- Ikeda, N.; Hayashida, O.; Kameda, H.; Ito, H.; Matsuda, T. Experimental study on thermal damage to dog normal brain. Int. J. Hyperth. 1994, 10, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Natah, S.S.; Srinivasan, S.; Pittman, Q.J.; Zhao, Z.; Dunn, J.F. Effects of acute hypoxia and hyperthermia on the permeability of the blood-brain barrier in adult rats. J. Appl. Physiol. 2009, 107, 1348–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harari, P.M.; Fuller, D.J.; Gerner, E.W. Heat shock stimulates polyamine oxidation by two distinct mechanisms in mammalian cell cultures. Int. J. Radiat. Oncol. 1989, 16, 451–457. [Google Scholar] [CrossRef]
- Alm, P.; Sharma, H.S.; Hedlund, S.; Sjoquist, P.O.; Westman, J. Nitric oxide in the pathophysiology of hyperthermic brain injury. Influence of a new anti-oxidant compound H-290/51. A pharmacological study using immunohistochemistry in the rat. Amino Acids 1998, 14, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sandor, N.; Walter, F.R.; Bocsik, A.; Santha, P.; Schilling-Toth, B.; Lener, V.; Varga, Z.; Kahan, Z.; Deli, M.A.; Safrany, G.; et al. Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS ONE 2014, 9, e112397. [Google Scholar] [CrossRef] [Green Version]
- van Vulpen, M.; Kal, H.B.; Taphoorn, M.J.; El-Sharouni, S.Y. Changes in blood-brain barrier permeability induced by radiotherapy: Implications for timing of chemotherapy? Rev. Oncol. Rep. 2002, 9, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Ou, G.; Mo, H.; Song, Y.; Kang, G.; Hu, Y.; Gu, X. Improved efficacy of chemotherapy for glioblastoma by radiation-induced opening of blood-brain barrier: Clinical results. Int. J. Radiat. Oncol. 2001, 51, 959–962. [Google Scholar] [CrossRef]
- Krueck, W.G.; Schmiedl, U.P.; Maravilla, K.R.; Spence, A.M.; Starr, F.L.; Kenney, J. MR assessment of radiation-induced blood-brain barrier permeability changes in rat glioma model. Am. J. Neuroradiol. 1994, 15, 625–632. [Google Scholar]
- Lemasson, B.; Serduc, R.; Maisin, C.; Bouchet, A.; Coquery, N.; Robert, P.; Le Duc, G.; Troprès, I.; Remy, C.; Barbier, E.L. Monitoring Blood-Brain Barrier Status in a Rat Model of Glioma Receiving Therapy: Dual Injection of Low-Molecular-Weight and Macromolecular MR Contrast Media 1. Radiology 2010, 257, 342–352. [Google Scholar] [CrossRef]
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Gebbers, J.O.; Laissue, J.A. Subacute neuropathological effects of microplanar beams of x-rays from a synchrotron wiggler. Proc. Natl. Acad. Sci. USA 1995, 92, 8783–8787. [Google Scholar] [CrossRef] [Green Version]
- Crosbie, J.C.; Svalbe, I.; Midgley, S.M.; Yagi, N.; Rogers, P.A.; Lewis, R.A. A method of dosimetry for synchrotron microbeam radiation therapy using radiochromic films of different sensitivity. Phys. Med. Biol. 2008, 53, 6861–6877. [Google Scholar] [CrossRef] [PubMed]
- Serduc, R.; Vérant, P.; Vial, J.-C.; Farion, R.; Rocas, L.; Rémy, C.; Fadlallah, T.; Brauer, E.; Bravin, A.; Laissue, J.; et al. In vivo two-photon microscopy study of short-term effects of microbeam irradiation on normal mouse brain microvasculature. Int. J. Radiat. Oncol. 2006, 64, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, A.; Potez, M.; Coquery, N.; Rome, C.; Lemasson, B.; Bräuer-Krisch, E.; Rémy, C.; Laissue, J.A.; Barbier, E.L.; Djonov, V.; et al. Permeability of Brain Tumor Vessels Induced by Uniform or Spatially Microfractionated Synchrotron Radiation Therapies. Int. J. Radiat. Oncol. 2017, 98, 1174–1182. [Google Scholar] [CrossRef]
- Serduc, R.; van de Looij, Y.; Francony, G.; Verdonck, O.; van der Sanden, B.; Laissue, J.; Farion, R.; Brauer-Krisch, E.; Siegbahn, E.A.; Bravin, A.; et al. Characterization and quantification of cerebral edema induced by synchrotron x-ray microbeam radiation therapy. Phys. Med. Biol. 2008, 53, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Aryal, M.; Park, J.; Vykhodtseva, N.; Zhang, Y.-Z.; McDannold, N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: Evaluation during tumor progression in a rat glioma model. Phys. Med. Biol. 2015, 60, 2511–2527. [Google Scholar] [CrossRef]
- Dréan, A.; Lemaire, N.; Bouchoux, G.; Goldwirt, L.; Canney, M.; Goli, L.; Bouzidi, A.; Schmitt, C.; Guehennec, J.; Verreault, M.; et al. Temporary blood–brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J. Neuro-Oncol. 2019, 144, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- McDannold, N.; Zhang, Y.; Supko, J.G.; Power, C.; Sun, T.; Peng, C.; Vykhodtseva, N.; Golby, A.J.; Reardon, D.A. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics 2019, 9, 6284–6299. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood–tumor and blood–brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013, 169, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, Z.I.; Tu, T.-W.; Sundby, M.; Qureshi, F.; Lewis, B.K.; Jikaria, N.; Burks, S.R.; Frank, J.A. MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 2018, 8, 4837–4855. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [Green Version]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.; Reinz, E.; Jenne, J.W.; Fatar, M.; Schmidt-Glenewinkel, H.; Hennerici, M.G.; Meairs, S. Reorganization of gap junctions after focused ultrasound blood-brain barrier opening in the rat brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 1394–1402. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.; Yang, P.H.; Kim, A.H. The effect of thermal therapy on the blood-brain barrier and blood-tumor barrier. Int. J. Hyperth. 2020, 37, 35–43. [Google Scholar] [CrossRef]
- Cao, Y.; Tsien, C.I.; Shen, Z.; Tatro, D.S.; Haken, R.K.T.; Kessler, M.L.; Chenevert, T.L.; Lawrence, T.S. Use of Magnetic Resonance Imaging to Assess Blood-Brain/Blood-Glioma Barrier Opening During Conformal Radiotherapy. J. Clin. Oncol. 2005, 23, 4127–4136. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Chen, X.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Miller, G.W.; Suk, J.S.; Hanes, J.; Price, R.J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, W.; Chen, Y.; Zhang, H.; Liu, C.; Deng, Z.; Sheng, Z.; Chen, J.; Liu, X.; Yan, F.; et al. Focused Ultrasound-Augmented Delivery of Biodegradable Multifunctional Nanoplatforms for Imaging-Guided Brain Tumor Treatment. Adv. Sci. 2018, 5, 1700474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Z.; Lin, Q.; Wong, H.L.; Shen, X.-T.; Yang, W.; Xu, H.-L.; Mao, K.-L.; Tian, F.-R.; Yang, J.-J.; Xu, J.; et al. Glioma-targeted therapy using Cilengitide nanoparticles combined with UTMD enhanced delivery. J. Control. Release 2016, 224, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, P.; Zhang, K.; Shi, Y.; Li, Y.; Li, C.; Lu, J.; Liu, Q.; Zheng, H. Manipulation of Mitophagy by “All-in-One” nanosensitizer augments sonodynamic glioma therapy. Autophagy 2019, 16, 1413–1435. [Google Scholar] [CrossRef]
- Hui, E.K.; Boado, R.J.; Pardridge, W.M. Tumor necrosis factor receptor-IgG fusion protein for targeted drug delivery across the human blood-brain barrier. Mol. Pharm. 2009, 6, 1536–1543. [Google Scholar] [CrossRef]
- McDannold, N.J.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- Fan, C.H.; Liu, H.L.; Ting, C.Y.; Lee, Y.H.; Huang, C.Y.; Ma, Y.J.; Wei, K.C.; Yen, T.C.; Yeh, C.K. Submicron-bubble-enhanced focused ultrasound for blood-brain barrier disruption and improved CNS drug delivery. PLoS ONE 2014, 9, e96327. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Huang, Y.; Hynynen, K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood–brain barrier in a rat model. Phys. Med. Biol. 2010, 55, 5251–5267. [Google Scholar] [CrossRef] [Green Version]



| Biochemical Modulation | Tumor Enriched Expression | Applied Drugs | FDA Approved | Tight Junction Effects | Vesicular Transport Effects | Clinical Stage | Refs |
|---|---|---|---|---|---|---|---|
| ATP-sensitive potassium channel (KATP channel) | Yes | Minoxidil sulfate | Yes | Occludin↓, Claudin-5↓ | Transport vesicles↑, Caveolin-1↑ | Preclinical | [57,58,59] |
| Calcium-activated potassium channel (KCa channel) | Yes | NS1619 | No | _ | Transport vesicles↑, Caveolin-1↑ | Preclinical | [60,61,62,63,64,65] |
| Phosphodiesterase 5 (PDE5) | Yes | Vardenafil (Levitra) | Yes | _ | Transport vesicles↑ | Preclinical | [66,67,68,69,70] |
| Bradykinin type 2 receptor (B2R) | Yes | Bradykinin and analogs | No | ZO-1↓, Occludin↓, Claudin-5↓ | Caveolin-1↑ | Clinical | [71,72,73,74,75,76,77,78,79,80,81] |
| Adenosine 2A receptor (A2AR) | Yes | Lexiscan | Yes | Occludin↓, Claudin-5↓ | _ | Clinical | [20,82,83,84,85] |
| Papaverine | _ | _ | No | Occludin↓, Claudin-5↓ | _ | Clinical | [86,87,88,89] |
| microRNAs | _ | miR-132-3p | No | _ | Caveolin-1↑ | Preclinical | [90] |
| Physical Modulation | Physical Source | Invasive or Noninvasive | Tight Junction Effects | Vesicular Transport Effects | Clinical Stage | Refs |
|---|---|---|---|---|---|---|
| Electromagnetic pulse (EMP) | Electromagnetic radiation | Noninvasive | ZO-1↓, Occludin↓, Claudin-5↓, MMP-2↑, MMP-9↑ | _ | Preclinical | [103,104,105,106,107,108] |
| Laser-induced thermal therapy (LITT) | Laser | Invasive, under anesthesia | _ | _ | Clinical | [109,110,111,112,113,114] |
| Radiotherapy: Synchrotron microbeam radiation therapy (MRT) | X-ray beams | Noninvasive | ZO-1↓, Claudin-5↓, beta-catenin↓ | _ | Preclinical | [115,116,117,118,119,120,121,122,123,124] |
| Focused ultrasound (FUS) | Ultrasonic waves with microbubbles | Noninvasive | Occludin↓, Claudin-1↓, Claudin-5↓ | Transport vesicles↑, Caveolin-1↑ | Clinical | [125,126,127,128,129,130,131,132,133,134,135,136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Shusta, E.V. Blood–Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics 2020, 12, 1085. https://doi.org/10.3390/pharmaceutics12111085
Luo H, Shusta EV. Blood–Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics. 2020; 12(11):1085. https://doi.org/10.3390/pharmaceutics12111085
Chicago/Turabian StyleLuo, Huilong, and Eric V. Shusta. 2020. "Blood–Brain Barrier Modulation to Improve Glioma Drug Delivery" Pharmaceutics 12, no. 11: 1085. https://doi.org/10.3390/pharmaceutics12111085