3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Preparation of 5-FU Coating Formulations
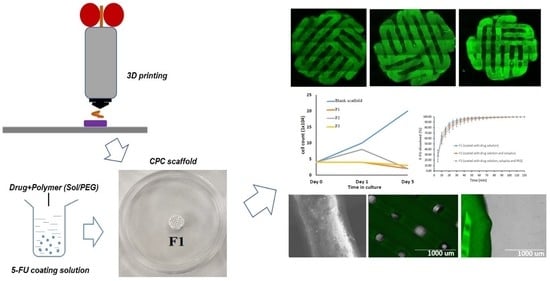
2.2.2. 3D Printing of the Scaffolds
2.2.3. Drug Coating for 3D Bio-Scaffolds
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Confocal Microscopy Analysis for 3D Bio-Scaffolds
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. X-Ray Powder Diffraction (XRD)
2.2.8. FTIR Studies
2.2.9. Texture Analysis
2.2.10. Cancer Cell Culture with 3D Bio-Scaffolds
2.2.11. Dissolution Studies and Drug Quantification
3. Results and Discussion
3.1. 3D Printed Scaffolds and Its Surface Morphology
3.2. Confocal Microscopy for 3D Bio-Scaffolds
3.3. DSC Analysis for Coating Ingredients
3.4. XRD Analysis of the Coated Scaffolds
3.5. FTIR Analysis
3.6. Texture Analysis
3.7. In Vitro Dissolution Studies
3.8. Cell Culture with 3D Bio-Scaffolds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lepowsky, E.; Muradoglu, M.; Tasoglu, S. Towards preserving post-printing cell viability and improving the resolution: Past, present, and future of 3D bioprinting theory. Bioprinting 2018, 11, e00034. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Jang, J.; Kang, H.-W. 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, T.G.; Jeong, J.; Yi, H.-G.; Park, J.W.; Hwang, W.; Cho, D.-W. 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink. Adv. Healthc. Mater. 2016, 5, 2636–2645. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.-S.; Gao, G.; Kim, Y.K. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I.T. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Lepowsky, E.; Tasoglu, S. 3D Printing for Drug Manufacturing: A Perspective on the Future of Pharmaceuticals. Int. J. Bioprinting 2018, 4, 119. [Google Scholar] [CrossRef]
- Kenny, S.M.; Buggy, M. Bone cements and fillers: A review. J. Mater. Sci. Mater. Med. 2003, 14, 923–938. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes – in vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; Bentley, K.L.D.M.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef] [PubMed]
- CAMEO Chemical Datasheet, Flurouracil. Available online: https://cameochemicals.noaa.gov/chemical/5005 (accessed on 19 October 2020).
- BASF, The Chemical Company, Soluplus, Technical Information. 2010. Available online: http://www.rumapel.com.ar/pharma_excipientes/ficha_tecnica/Soluplus.pdf (accessed on 10 November 2020).
- BASF SE 2020. Soluplus—For Better Solubility and Bioavailability. Available online: https://pharmaceutical.basf.com/en/Drug-Formulation/Soluplus.html (accessed on 19 September 2020).
- Kanmani, P.; Lim, S.T. Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr. Polym. 2013, 97, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M. 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Sakai, S.; Yoshii, A.; Sakurai, S.; Horii, K.; Nagasuna, O. Silk fibroin nanofibers: A promising ink additive for extrusion 3D bioprinting. Mater. Today Bio 2020, 8, 100078. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Kim, W.; Kim, G.H. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr. Polym. 2020, 250, 116914. [Google Scholar] [CrossRef]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair. Polymers 2019, 11, 1924. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Belleghem, S.; Torres, L.; Santoro, M.; Mahadik, B.; Wolfand, A.; Kofinas, P.; Fisher, J. Hybrid 3D Printing of Synthetic and Cell-Laden Bioinks for Shape Retaining Soft Tissue Grafts. Adv. Funct. Mater. 2020, 30, 1907145. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.F.; Schweizer, P.M. Liquid Film Coating: Scientific Principles and Their Technological Implications; Chapman & Hall: New York, NY, USA, 1997; pp. 401–536. [Google Scholar]
- Hong, Y. Electrospun Fibrous Polyurethane Scaffolds in Tissue Engineering. In Advances in Polyurethane Biomaterials; Woodhead Publishing: Sawston/Cambridge, UK, 2016; pp. 543–559. [Google Scholar]
- Maniruzzaman, M.; Douroumis, D. An in-vitro-in-vivo taste assessment of bitter drug: Comparative electronic tongues study. J. Pharm. Pharmacol. 2014, 67, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-On-Powder 3D Printing of Tablets with an Anti-Cancer Drug, 5-Fluorouracil. Pharmaceutics 2019, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Akbari, J.; Adrangui, M.; Farid, D.; Siahi-Shadbad, M.R.; Saeedi, M.; Nokhodchi, A. The effects of various factors on the release rate of a poorly solubledrug (carbamazepine) from hydroxypropyl methylcellulose matrices. S.T.P. J. Pharma. Sci. 2000, 10, 473–478. [Google Scholar]











| Formulation Code | 5-FU (g) | DI Water (ml) | Soluplus (g) | PEG 6000 (g) |
|---|---|---|---|---|
| F1 | 0.5 | 20 | 0 | 0 |
| F2 | 0.5 | 20 | 0.50 | 0 |
| F3 | 0.5 | 20 | 0.25 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. https://doi.org/10.3390/pharmaceutics12111077
Wu Y, Woodbine L, Carr AM, Pillai AR, Nokhodchi A, Maniruzzaman M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics. 2020; 12(11):1077. https://doi.org/10.3390/pharmaceutics12111077
Chicago/Turabian StyleWu, Yan, Lisa Woodbine, Antony M. Carr, Amit R. Pillai, Ali Nokhodchi, and Mohammed Maniruzzaman. 2020. "3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery" Pharmaceutics 12, no. 11: 1077. https://doi.org/10.3390/pharmaceutics12111077