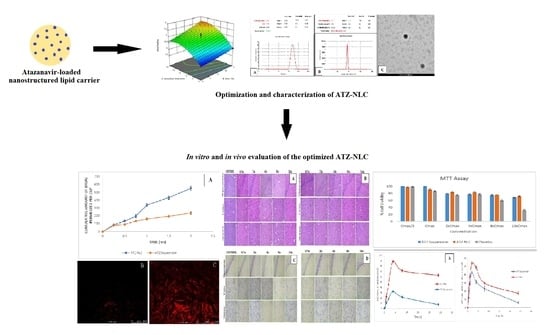
1. Introduction
Acquired immunodeficiency syndrome (AIDS), a pandemic and perhaps the deadliest malady has claimed more than 39 million lives up until the year 2014. Currently, 37.3 million people have active HIV bringing about yearly demise of around 1.2 million individuals [
1]. As per the World Health Organization (WHO), nearly 1.8 million people became newly infected and 9.4 million people died in 2017 due to AIDS. Further, as per the UNAIDS (The Joint United Nations Program on HIV/AIDS) data, India in 2017 witnessed 2.1 million individuals affected with AIDS, 69,000 deaths, 88,000 new HIV infections, 0.2% adult HIV prevalence, and 56% adults and 33% children were on antiretroviral therapy [
2]. Thus, being a fatal disease of pandemic extents with continually rising global burden and distressing health-related and socioeconomic effects, AIDS has become a serious cause of concern worldwide.
The causative organism of AIDS is the human immunodeficiency virus (HIV), which debilitates the immune system by triggering the radical loss and dysregulation of the macrophages and CD4+T lymphocytes [
3]. The mortality and morbidity rate linked to AIDS has shown a dramatic depreciation with the dawn of highly active antiretroviral therapy (HAART) [
4]. The chronic administration of HAART, which is a combination of antiretroviral (ARV) drugs, proved quite effective in diminishing the viral burden and controlling its replication and transmission thereby assuring a long and productive life to the affected patients. However, the therapy lacked in offering a complete annihilation of the virus from the body owing to the existence of HIV in the anatomical reservoirs like the central nervous system (CNS), lymphatic system, lung, and liver. The virus in these reservoirs becomes latent and the presence of anatomical barriers limit the access to ARVs [
5,
6]. The ineptitude of the systemically administered ARVs to traverse the blood–brain barrier (BBB) makes the brain one of the predominant HIV reservoirs in the affected patients [
7].
NeuroAIDS is consequently turning into a very common complication causing adverse neurological dysfunctions in the HIV-tainted patients. HIV primarily targets microglia, mononuclear macrophages and perivascular macrophages in the brain. The virus enters the brain via infected circulating monocytes and replicates itself thereby prompting reinfection and inducing the drug resistance [
8]. It is realized that the vast majority of the currently available ARVs are unable to cross the impregnable BBB while some possessing this ability exhibit deficient concentration in the brain leading to their therapeutic failure [
9]. Some of the other impediments in the path of ARVs moving to the brain include the absence of ideal physicochemical properties of ARVs, existence of efflux transporters like P-glycoprotein (P-gp) and metabolizing enzymes on BBB [
5]. This entail administration of high doses, which is certainly not a practical solution as it prompts serious side effects like hematological intolerance when administered for a longer span [
6]. This warrants identification of a suitable drug delivery system, which assures the successful delivery of ARVs into the brain for an effective management of AIDS.
Nanostructured lipid carriers (NLCs) appear to be a profound substitute for targeting ARVs into the brain. These lipid nanoparticles are known to experience lymphatic absorption, which ensures prolonged blood circulation of the ARVs and effective brain targeting. This will likewise guarantee the accessibility of the drugs at the viral sanctuaries (lymphatic system) and also avoid first-pass hepatic metabolism [
10]. In the recent years, NLCs have encountered considerable attention for brain targeting, which can be attributed to the rapid brain uptake, inhibition of P-gp-mediated drug efflux, controlled and sustained drug release, presence of physiological lipids, biocompatibility, biodegradability, long-term storage stability, and less toxicity [
11]. Ease of preparation, cost effectiveness, and industrial scalability are other advantages of NLC. Additionally, the presence of both liquid and solid lipid in the NLC steers to high drug entrapment and improved drug loading, which makes these carriers a promising contender for delivery of therapeutics past the brain [
12].
The lipid digestion of NLC by the pancreatic enzymes present in the duodenum leads to the formation of monoglycerides and fatty acids, which further form micelles after being enveloped by bile salts. After these micelles reach the enterocytes, triglycerides are formed in the intestinal cells. On aggregation with phospholipids and cholesterol, these lipid constituents form chylomicrons, which are unable to cross the blood capillaries due to their large size [
13]. The chylomicrons undergo lymphatic uptake, followed by the glymphatic system of the brain, where receptor mediated transcytosis (RMT) is the mechanism utilized by NLC [
14,
15]. After administration of nanoparticles, their concentration in the brain depends on factors like plasma-protein binding, permeability across BBB, efflux by efflux transporters, enzymatic metabolism, and plasma–concentration time curve [
16]. Further, the endothelial cells bear a negative surface charge, which is a crucial component of the defense system of BBB, as it regulates the permeation of the positively charged molecules across BBB [
17]. Atazanavir (ATZ), an azapeptide derivative is a protease inhibitor utilized for the treatment therapy of HIV. It acts selectively on HIV-1, attaching to the protease active site of the HIV-1 protease enzyme and inhibiting its activity [
18]. ATZ is a BCS class II drug with low aqueous solubility (0.003 mg/mL) and high permeability (log P 4.11). The drug suffers from extensive hepatic metabolism, and undergoes an efflux by P-gp efflux transporters, consequently leading to low oral bioavailability and lesser brain biodistribution. Moreover, traversing through the BBB becomes more challenging owing to the extensive plasma protein binding and high molecular weight [
19]. Although a solid lipid nanoparticle (SLN) and self-nanoemulsifying drug delivery system (SNEDDS) of ATZ for brain targeting have been prepared earlier, none of the research report formulation of ATZ-loaded NLC [
19,
20]. NLCs are reported to overcome the several disadvantages of SLN like poor drug loading (due to the absence of liquid lipid) and drug expulsion on prolonged storage. Moreover, in SLN, only up to 30% lipid can be used as beyond 30%, it leads to the formation of bicoherent systems. For NLC, the lipid ratio allowed in the formulation is up to 95%, which is much higher than SLN. Based on these disadvantages, the application of SLN is limited and NLCs are more favorable delivery system [
9,
21]. Further, SNEDDS of ATZ has also been prepared, but limitations like gastrointestinal irritation due to large amount of surfactants, precipitation of the drug in vivo, and instability due to variation in pH and temperature hinders its wide applicability [
22,
23]. NLC are devoid of such disadvantages, and thus forms the basis of this research
Accordingly, in the present investigation, the potential of NLCs was examined for augmenting the oral bioavailability and brain distribution of ATZ following its oral administration. The formulation was prepared using the quality by design (QbD)-based approach, which helps in examining all the factors included in formulating NLC and the interactions among such factors, so that a high quality formulation is obtained. The prepared formulation was subjected to in vitro and in vivo evaluations. The formulated NLCs were analyzed for brain targeting efficiency in Wistar rats. The findings suggested NLC to be a prospective carrier for the effective management of AIDS.
2. Materials and Methods
2.1. Materials
Atazanavir was obtained as a generous gift from Sun Pharmaceutical Industries Limited (Gurgaon, India). Labrafil® M1944CS, Labrafil® M2125CS, LabrafacTM WL1349, LabrafacTM PG, LauroglycolTM 90, CapryolTM PGMC, Compritol® 888ATO, Precirol® ATO 5, Solutol HS15®, Glyceryl monostearate, Gelucire® 44/14, and Gelucire® 43/10 were acquired by Gattefosse (Saint Priest, France). Capmul® PG-12 EP/NF, Captex® 100, and Captex® 300 were obtained from Abitech Corporations (Cleveland, OH, USA). The surfactants like span 20, tween 80, and tween 20 were procured from Merck (Hohenbrunn, Germany). Poloxamer 188, Poloxamer 407, and Cremophor RH 40 were acquired from BASF (Mumbai, India). Sesame oil, canola oil, and castor oil were acquired from the central drug house (Mumbai, India). Water, methanol, and acetonitrile for HPLC were procured from Fischer Scientific Co. (Mumbai, India). Purified water used for all experiments was obtained from Milli Q Plus (Millipore, MA, USA). Rest of the chemicals and reagents were procured from S.D. Fine Chemicals Ltd. (Mumbai, India). Freshly prepared buffer solutions were used for all experiments.
2.2. Animals Used
Adult albino Wistar rats of weight 200–250 g obtained from the Central Animal House, Jamia Hamdard, New Delhi, India were used. The animals were housed in cages kept under set laboratory specifications of 25 ± 2 °C temperature and 55% ± 5% relative humidity with a 12 h light–dark cycle. The animals had unrestricted access to standard diet and water. The investigation was carried out as per the protocols certified by Institutional Animal Ethics Committee of Jamia Hamdard, New Delhi, India (protocol approval number: 1519). The committee is listed in the Committee for the Purpose of Control and Supervision of Experiments on Animals (173/GO/Re/S/2000/CPCSEA).
2.3. Screening of Lipids and Surfactants
For selecting the liquid lipid, various lipids and oils like Captex
® 100, Captex
® 300, Capryol
TM PGMC, Capmul
® PG-12 EP/NF, Labrafac
TM WL1349, Labrafac
TM PG, and Lauroglycol
TM 90, Labrafil
® M1944CS, Labrafil
® M2125CS, castor oil, canola oil, and sesame oil were screened for solubility of ATZ. To each vial comprising 1 mL each of different liquid lipids, an excess quantity of ATZ was put in. The vials were equilibrated for 72 h in an isothermal shaker (25.0 ± 0.5 °C) and the centrifugation of these samples were done for 30 min at a speed of 5000 rpm by a high-speed centrifuge (Remi Instruments, Delhi, India). The supernatant was isolated, diluted in methanol, and analyzed at λ
max of 247 nm by the UV-spectrophotometer (UV 1700, Shimadzu, Japan). The lipids presenting highest solubility was chosen for preparing NLC [
24].
The solid lipids (Gelucire
® 44/14, and Gelucire
® 43/10, Precirol
® ATO 5, Compritol
® 888ATO, and glyceryl monostearate) were added in small increments to the vials, each containing 10 mg of ATZ. The vials were heated at above 5 °C beyond the respective melting points of solid lipid. The amount of each solid lipid utilized for dissolving ATZ was determined. The lipid dissolving the drug completely in the minimum amount was chosen for NLC preparation. The end point was considered as the absence of any undissolved drug in the vials [
10].
The liquid and solid lipids chosen for preparing NLC were blended in various proportions (9:1, 8:2, 7:3, and 6:4), melted and cooled down to room temperature. The blends were visually examined for uniformity, clarity, turbidity, and phase separation. The solid–liquid binary lipid (SLB) mixture showing good miscibility without any indication of phase separation or turbidity was selected for the design of NLC [
25].
For selecting surfactants, 100 mg of SLB was placed in a glass vial and methylene chloride (3 mL) was put in to dissolve it. For each of the 5% solutions of various surfactants (Span 20, Poloxamer 188, Poloxamer 407, Cremophor RH 40, tween 80, and tween 20), 10 mL were prepared. Further, the SLB blend was put in the surfactant solution under magnetic stirring (Remi Instruments, Mumbai, India). The mixtures were kept at 40 °C to remove the organic phase and then diluted to 10-folds using Milli-Q water. UV-spectrophotometer was utilized for recording the percentage transmittance of each sample at 510 nm [
26].
2.4. Quality by Design (QbD) Approach
The authors harnessed QbD for fabrication of ATZ-NLC so that a safe, effective, patient centric formulation evincing the desired characteristics can be produced. For this approach, quality target product profile (QTPP) was established. The process also involved identification of critical process parameters (CPPs) and critical material attributes (CMAs), which bear an influence on critical quality attributes (CQAs). Limits are defined for CQAs as they ascertain the final characteristics and quality of the formulation [
27].
Table 1 exhibits the different aspects of QTPP and CQA while the risk assessment was established using the Ishikawa (fish-bone) diagram (Minitab 17 software, M/s Minitab Inc., Philadelphia, USA as shown in
Figure 1.
2.5. Preparation of ATZ-NLC
ATZ-NLCs were developed by melt emulsification followed by the probe sonication method. The SLB containing Precirol ATO 5 (solid lipid) and Lauroglycol 90 (liquid lipid) in the ratio of 70:30 was melted at 70 °C. ATZ (3 mg) was then put into the melted SLB and allowed to dissolve. The aqueous phase contained Cremophor RH 40 (3%
w/v) solubilized in 10 mL of water and kept at the same temperature. The hot aqueous mixture was then transferred gradually to the molten lipid phase and stirring was continued for 30 min at 500 rpm. This pre-emulsion was then treated for 5–15 min on an ice bath using a probe sonicator (Labsonic
® M) at 40% amplitude and 6 cycles to obtain NLC formulation of ATZ [
28].
2.6. Optimizing ATZ-NLC by the Box–Behnken Design (BBD)
ATZ-NLC was optimized using response surface methodology (RSM), which quantifies the relationship amidst controllable independent variables and the responses obtained. A three-factor three-level BBD utilizing Design-Expert 11 software (Stat-Ease Inc., Minneapolis, MN, USA) was utilized to examine the effects of lipid concentration, surfactant concentration, and time of sonication on particle size, entrapment efficiency, and drug loading of NLCs.
Table 2 represents the various levels (low, medium, and high) used for the independent and dependent variables. BBD yielded 17 experimental runs and a quadratic Equation (1), which is as given under:
where
Y = measured response for each factor level combination,
= intercept,
= regression coefficients,
= coded levels of independent variables, and
and
(
i,
j = 1, 2 or 3) = interaction and quadratic coefficients of the observed experimental values [
29].
2.7. Lyophilization of Optimized ATZ-NLC
The aqueous dispersions of ATZ-NLC was mixed with 1%
w/
v mannitol (cryoprotectant) and stored at −80 °C for 24 h. The freeze dryer (Metrex Scientific Instruments Pvt. Ltd., Delhi, India) was used for the lyophilization of the samples to obtain free-flowing dry powder for further characterization (like differential scanning calorimetry (DSC) and FTIR) [
25].
2.8. Evaluation of Optimized ATZ-NLC
2.8.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential (ZP)
These parameters were estimated by Malvern Zetasizer (Nano-ZS; Malvern Instruments, Malvern, UK). Before the estimations, the samples were diluted using double distilled water (1:10) to yield an appropriate scattering intensity. ZP was measured using disposable polystyrene cells at 25 °C and at an angle of 90° [
30].
2.8.2. Drug Entrapment Efficiency (%EE) and Drug Loading Capacity (%LC)
The aqueous dispersions of ATZ-NLC were diluted with 0.1 N HCl to adjust its pH in the range of 1.5–2. This will cause aggregation of the NLCs thus making the separation easier during centrifugation. The samples were then centrifuged at 30,000 rpm for 30 min by means of a high-speed centrifuge (Sigma-3K30, Osterode am Harz, Germany). Isolation of the supernatant was done, suitably diluted with methanol, and analyzed via UV spectrophotometer. %EE and %LC are determined using the following Equations (2) and (3):
where,
Wt = weight of initial drug added,
Wf = weight of unencapsulated drug present in supernatant, and
Wl = total weight of liquid lipid and solid lipid [
25].
2.8.3. Transmission Electron Microscopy (TEM)
ATZ-NLC was analyzed for particle size and surface morphology using TEM (CM 200, Philips Briarcliff Manor, NY, USA) operated at a voltage of 200 kV. A drop of aqueous dispersion of each sample (ATZ-NLC and placebo) was positioned on a carbon film-covered copper grid (400-mesh), which was followed by negative staining with 1% phosphotungstic acid. The excess liquid was removed, and the copper grid was dried out at room temperature. Later, the specimens were then analyzed under the microscope [
31].
2.8.4. Differential Scanning Calorimetry (DSC)
The DSC examination of ATZ and optimized ATZ-NLC was performed using DSC (Perkin Elmer, Pyris 6 DSC, USA) to understand their melting behavior and crystallinity. The instrument was operated from 30 to 400 °C, at nitrogen purging of 40 mL/min and heating rate of 10 °C/min. 1-2 mg of each samples was sealed in aluminum pans, equilibrated at 25 °C and exposed to DSC analysis. An empty sealed aluminum pan was treated as the reference [
32].
2.9. In Vitro Release Study
The drug release from the suspension and optimized NLC of ATZ was assessed in 0.1 N HCl pH 1.2, phosphate buffer saline (PBS) pH 6.8 and PBS pH 7.4 (simulating CSF) using the dialysis technique. Typically, 3 mL each of drug suspension and ATZ-NLC, corresponding to 100 mg ATZ was put separately in activated dialysis bags (mol. wt. 12,000–14,000 Da; Hi Media, Mumbai, India). The bags were secured at both the ends and were then suspended in 100 mL of each media kept at 37 ± 0.5 °C and stirred at 50 rpm. About 2 mL of samples were drawn from each media at definite time intervals (0.25, 0.5, 1, 2, 4, 8, 12, and 24 h) and substituted with fresh dissolution medium at that same time-point to maintain sink conditions. The drug content was estimated for the withdrawn samples utilizing UV spectrophotometer at λ
max of 247 nm. The results were incorporated to various kinetic models like zero order, first order, Higuchi, and Korsmeyer–Peppas to ascertain the drug release mechanism [
33].
2.10. Quantification of ATZ by HPLC
As per the formerly published method by Konidala et al., a reverse phase high performance liquid chromatographic method (HPLC) with the UV detector method was utilized for the estimation of ATZ in in vitro and in vivo studies. The chromatography was performed utilizing C18 column with specifications of 250 mm × 4.6 mm × 5 µm at 255 nm. Class VP software was utilized for the area calculation of the chromatograms. The method was validated and found to be rapid, simple, sensitive, precise, and accurate. The mobile phase comprised of water: acetonitrile (20:80
v/v) with pH adjusted to 3.0 with glacial acetic acid. The mobile phase was propelled at a flow rate of 1 mL/min. Calibration curve was plotted ranging from 100 to 1000 ng/mL. The retention time for ATZ was obtained at 3.9 min. The detection and quantification limit were assessed to be 67.16 ng/mL and 203.53 ng/mL [
34].
2.11. Ex Vivo Permeation Study
The study was carried out using small intestine isolated from the Wistar rats, which were fasted overnight and sacrificed using the CO
2 inhalation technique. The intestinal segments were surgically isolated and washed with the Tyrode solution (composed of 15 mM glucose, 136.9 mM sodium chloride, 11.90 mM sodium bicarbonate, 4.2 mM sodium dihydrogen phosphate, 2.7 mM potassium chloride, 1.2 mM calcium chloride, and 0.5 mM magnesium chloride). They were then into segments each measuring 6–7 cm. One end of each segment was tied to form a sac, filled with 1 mL each of NLC and drug suspension and then ligated at the other end. The sacs were suspended for 2 h in 100 mL of Tyrode solution preheated at a temperature of 37 ± 0.5 °C and provided with continuous aeration. Aliquots of 1 mL from each beaker were drawn at preset time-points and replenished with the equal volume of preheated Tyrode solution. The extent of drug permeated beyond the intestinal barrier was estimated by using HPLC [
35].
2.12. Confocal Laser Scanning Microscopy (CLSM)
This experiment was to assess whether ATZ-NLC crossed BBB and accumulated in the brain upon oral administration. The NLC formulation and drug suspension were treated with rhodamine 123 and administered to the rats via oral feeding sonde. The labeling with the dye was done at the time of the formulation preparation by adding the dye to the melted lipid phase. The rats were sacrificed via the CO
2 inhalation method and their brains were isolated and washed with PBS pH 7.4. The brain samples were excised into thin sections and examined using CLSM (TCS SP5II, Leica Microsystem Ltd., Wetzlar, Germany). The fluorescence signal was perceived at several depths of the brain indicating the depth of penetration of the formulation [
1].
2.13. In Vitro Cell Viability Study
The study was executed in Neuro-2a (ATCC CCL-131) brain-derived neuroblastoma cells to assess the cytotoxic activity. About 1 × 10
4 cells were plated in each well of a 96-well plate for 24 h with Dulbecco’s modified Eagle’s medium (DMEM) accompanied with 10% fetal bovine serum, 100 μg/mL streptomycin, 100 μg/mL penicillin, and 2 mmol/l L-glutamine. Incubation of the cells was done at atmospheric conditions of 37 °C, 100% relative humidity with 95% O
2/5% CO
2. The cells were treated for 18 h with samples (ATZ suspension, ATZ-NLC, placebo NLC) of varying concentrations according to the C
max of the drug. Thereafter, 10%
w/v methylthiazoletetrazolium (MTT; 5 mg/mL) was used for the cell treatment, which were then incubated for 4 h at 37 °C. Lastly, the treatment of the cells was done with 10%
w/v dimethylsulfoxide so that the formazan crystals can be solubilized. The plates were then read using scanning multiwall spectrophotometer and absorbance was recoded at 570 nm [
36]. The % cell viability was computed as per the following Equation (4):
2.14. Histopathological Examination
The brain was isolated from the rats administered with ATZ-suspension and ATZ-NLC. The brain tissues were fixed for 24 h in 10% neutral buffered formalin, which were then washed with water and dehydration by alcohol. The samples were cleaned up using xylene and embedded in paraffin bees wax blocks for an additional 24 h at 56 °C. Slices of thickness 5 μm were cut transversally by a slide microtone and stained. The slices were isolated on glass slides, deparaffinized and then stained with hematoxylin and eosin (H&E) staining and cresyl violet (CV) staining dyes. The brain sections of hippocampus, cortex, and striatum were examined for any histological alterations using the fluorescence motic microscope (Motic AE31) enabled with infinity analyze software [
4].
2.15. In Vivo Study
For the conduction of the pharmacokinetic studies, the rats were distributed into three groups namely the (1) control group, (2) group administered with ATZ suspension, and (3) group receiving ATZ-NLC with each group containing 15 animals. Using the 18-gauge oral feeding needle, group 1 received saline while group second and third received drug suspension and NLC formulation respectively at the required animal dose of 10.27 mg/kg body weight. The rats were sacrificed using the CO2 inhalation method at designated time-points (0.5, 1, 2, 4, 8, and 24) and blood and brain were isolated.
2.15.1. Isolation and Extraction of Plasma and Brain Samples
The blood specimens at each time-point were collected in tubes containing the EDTA anticoagulant. These specimens were then centrifuged at 5000 rpm for 15 min to collect the plasma, which was kept at −20 °C until the day of evaluation. From each sample, 500 μL of plasma was withdrawn and 500 μL of ethyl acetate was added and further centrifuged at 10,000 rpm for 10 min. The supernatant was isolated from each sample in fresh eppendorfs and evaporated to dryness. The residue left was reconstituted with the mobile phase (100 μL), from which 20 μL was injected into the HPLC column [
37].
For the estimation of drug content in the rat brain at each time-point, the whole brain from each rat was isolated, washed with isotonic PBS pH 7.4, and thawed until analysis. Thereafter, 1 g of brain was weighed from each sample and 2 mL of PBS pH 7.4 was put in each vial followed by their homogenization. The samples were then extracted with ethyl acetate, vortexed, and centrifuged at 5000 rpm for 15 min. The supernatants were collected, and the samples were extracted again. The supernatants were united and later evaporated to dryness. The residue was reconstituted with 200 μL of mobile phase and filtered with a 0.45 μm nylon membrane filter. Of the filtered sample 20 μL was then injected into HPLC for analysis [
38].
2.15.2. Data Analysis
The various pharmacokinetic parameters were computed using the using pharmacokinetic software (PK Functions for Microsoft Excel, Pharsight Corporation, Mountain View, CA, USA). The maximum plasma concentration of ATZ (Cmax) and the time required to achieve the maximum concentration (Tmax) were noted from the actual plasma profiles.
2.16. Stability Studies
The formulation ATZ-NLC was preserved in a glass vial and stored for three months at the room temperature (25 ± 2 °C/60% ± 5% RH). The formulation was analyzed at specific time-points of 0, 30, 60, and 90 days for any formation of precipitate, change in physical appearance, particle size, polydispersity index (PDI), and drug content [
33].
2.17. Statistical Analysis
The outcomes of all studies were replicated thrice and expressed as mean ± SD. The results were compared and examined using a one-way analysis of variance (ANOVA) using Graph Pad Instat 3 (GraphPad InStat Software, Inc., San Diego, CA, USA). The findings with a statistical difference of p < 0.05 were deemed to be significant.