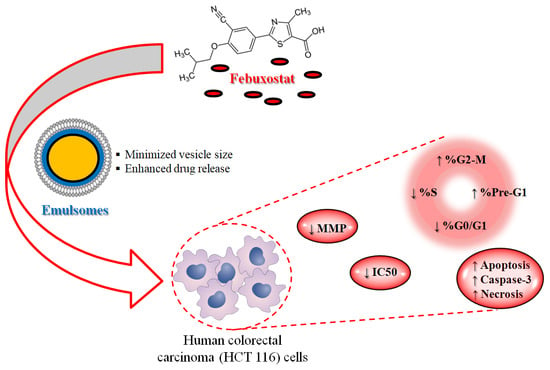
The Encapsulation of Febuxostat into Emulsomes Strongly Enhances the Cytotoxic Potential of the Drug on HCT 116 Colon Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Design of FBX-EMLs
2.3. Preparation of FBX-EMLs
2.4. Measurement of Vesicle Size
2.5. Optimization of FBX-EMLs
2.6. In Vitro FBX Release from the Optimized EMLs Formula
2.7. Determination of IC50 by MTT Assay
2.8. Cell Cycle Analysis
2.9. Annexin V Staining
2.10. Caspase-3 Assay
2.11. Mitochondrial Membrane Potential (MMP)
2.12. Statistical Analysis
3. Results
3.1. Experimental Design of FBX-EMLs
3.1.1. Fit Statistics for Sequential Model Selection and Validation
3.1.2. Statistical Analysis for the Effect of Variables on Particle Size (Y)
3.2. Optimization of SMV-EMLs
3.3. In Vitro FBX Release from the Optimized EMLs Formula
3.4. Optimized FBX-EML Formulation Shows the Lowest IC50 Value
3.5. FBX-EMLs Treatment Inhibits the Proliferation of HCT 116 Cells
3.6. The Encapsulation of FBX into EMLs (FBX-EMLs) Strongly Enhances the Pro-Apoptotic Potential of the Drug
3.7. Caspase-3 Activity Increases Following the Treatments with the Free Drug (FBX-R) and the Optimized Formulation (FBX-EMLs)
3.8. The FBX Ability to Decrease the MMP is Enhanced When Encapsulated into the EMLs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, B.; Prasad, S.; Yadav, V.R.; Aggarwal, B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer 2012, 64, 173–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Powers, S.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016, 529, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [Green Version]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Khosravan, R.; Grabowski, B.A.; Wu, J.T.; Joseph-Ridge, N.; Vernillet, L. Pharmacokinetics, pharmacodynamics and safety of febuxostat, a non-purine selective inhibitor of xanthine oxidase, in a dose escalation study in healthy subjects. Clin. Pharm. 2006, 45, 821–841. [Google Scholar] [CrossRef]
- Loeb, J.N. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972, 15, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Du, R.; Song, P.; Zhong, T.; Zhang, W.; Zhao, Y.; Wang, C.; Zhang, X.; Zhang, Q. The preparation and characteristics of febuxostat sio 2 solid dispersions. J. Chin. Pharm. Sci. 2014, 23, 463–470. [Google Scholar] [CrossRef]
- Asif, U.; Sherwani, A.K.; Akhtar, N.; Shoaib, M.H.; Hanif, M.; Qadir, M.I.; Zaman, M. Formulation development and optimization of febuxostat tablets by direct compression method. Adv. Polym. Technol. 2016, 35, 129–135. [Google Scholar] [CrossRef]
- Bisht, M.; Bist, S.S. Febuxostat: A novel agent for management of hyperuricemia in gout. Indian J. Pharm. Sci. 2011, 73, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Kawai, Y.; Kiguchi, T.; Okamoto, M.; Kaneko, M.; Maemondo, M.; Gemba, K.; Fujimaki, K.; Kirito, K.; Goto, T.; et al. Efficacy and safety of febuxostat for prevention of tumor lysis syndrome in patients with malignant tumors receiving chemotherapy: A phase iii, randomized, multi-center trial comparing febuxostat and allopurinol. Int. J. Clin. Oncol. 2016, 21, 996–1003. [Google Scholar] [CrossRef]
- Alfaifi, M.Y.; Shati, A.A.; Elbehairi, S.E.I.; Fahmy, U.A.; Alhakamy, N.A.; Md, S. Anti-tumor effect of peg-coated plga nanoparticles of febuxostat on a549 non-small cell lung cancer cells. 3 Biotech 2020, 10, 133. [Google Scholar] [CrossRef]
- Kamel, B.; Graham, G.G.; Williams, K.M.; Pile, K.D.; Day, R.O. Clinical pharmacokinetics and pharmacodynamics of febuxostat. Clin. Pharm. 2017, 56, 459–475. [Google Scholar] [CrossRef] [Green Version]
- Amselem, S.; Yogev, A.; Zawoznik, E.; Friedman, D. Emulsomes, a Novel Drug Delivery Technology. In Proceedings of the International Symposium on Controlled Release of Bioactive Materials, Nice, France, 27–30 June 1994; pp. 1368–1369. [Google Scholar]
- Amselem, S.; Friedman, D. Solid Fat Nanoemulsions. Google Patents, US Patent no 5,662,932, 2 September 1997. [Google Scholar]
- Vyas, S.P.; Subhedar, R.; Jain, S. Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. J. Pharm. Pharmacol 2006, 58, 321–326. [Google Scholar] [CrossRef]
- Pal, A.; Gupta, S.; Jaiswal, A.; Dube, A.; Vyas, S.P. Development and evaluation of tripalmitin emulsomes for the treatment of experimental visceral leishmaniasis. J. Liposome Res. 2012, 22, 62–71. [Google Scholar] [CrossRef]
- McKenney, J.M. Pharmacologic characteristics of statins. Clin. Cardiol. 2003, 26, Iii32–Iii38. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.; Müller, R. Solid lipid nanoparticles (sln) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Mishra, N.; Mehta, A.; Vyas, S.P. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int. J. Pharm. 2009, 380, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ucisik, M.H.; Sleytr, U.B.; Schuster, B. Emulsomes meet s-layer proteins: An emerging targeted drug delivery system. Curr. Pharm. Biotechnol. 2015, 16, 392–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, N.; Wang, L. In vitro human cell line models to predict clinical response to anticancer drugs. Pharmacogenomics 2015, 16, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, P.; Serviss, J.T.; Lundbäck, T.; Bancaro, N.; Mazurkiewicz, M.; Kolosenko, I.; Yu, D.; Haraldsson, M.; D’Arcy, P.; Linder, S.; et al. A drug screening assay on cancer cells chronically adapted to acidosis. Cancer Cell Int. 2018, 18, 147. [Google Scholar] [CrossRef]
- Mahajna, S.; Kadan, S.; Tietel, Z.; Saad, B.; Khasib, S.; Tumeh, A.; Ginsberg, D.; Zaid, H. In vitro evaluation of chemically analyzed hypericum triquetrifolium extract efficacy in apoptosis induction and cell cycle arrest of the hct-116 colon cancer cell line. Molecules 2019, 24, 4139. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.F.; Lai, G.Y.; Chen, C.H.; Chiu, C.C.; Hung, S.W.; Chang, C.F. 6,7-dihydroxy-2-(4′-hydroxyphenyl)naphthalene induces hct116 cell apoptosis through activation of endoplasmic reticulum stress and the extrinsic apoptotic pathway. Drug Des. Devel. Ther. 2019, 13, 1609–1621. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, U.A. Quantification of simvastatin in mice plasma by near-infrared and chemometric analysis of spectral data. Drug Des. Devel. Ther. 2016, 10, 2507–2513. [Google Scholar] [CrossRef] [Green Version]
- Mukthinuthalapati, M.A.; Bandaru, S.P.; Bukkapatnam, V.; Mohapatro, C. Development and validation of a stability-indicating rp-hplc method for the determination of febuxostat (a xanthine oxidase inhibitor). J. Chromatogr. Sci. 2013, 51, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Distefano, D.A.; Parlascino, P.; Fresta, C.G.; Lazzarino, G.; Lunte, S.M.; Nicoletti, V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell Biochem. 2017, 425, 85–93. [Google Scholar] [CrossRef]
- Awan, Z.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Ibrahim, T.S.; Asfour, H.Z.; Al-Rabia, M.W.; Alfarsi, A.; Alhakamy, N.A.; Abdulaal, W.H.; Al Sadoun, H.; et al. The enhanced cytotoxic and pro-apoptotic effects of optimized simvastatin-loaded emulsomes on mcf-7 breast cancer cells. Pharmaceutics 2020, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Wu, Y.J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer effects of sinulariolide-conjugated hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.H. Febuxostat: A novel non-purine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in gout. Recent Pat. Inflamm. Allergy Drug Discov. 2007, 1, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-R.; Zhang, L. Simultaneous enhancements of solubility and dissolution rate of poorly water-soluble febuxostat via salts. J. Mol. Struct. 2017, 1137, 328–334. [Google Scholar] [CrossRef]
- Kumar, R.; Seth, N. Emulsomes: An emerging vesicular drug delivery system. J. Drug Deliv. Ther. 2013, 3, 133–142. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.K.; Srinivas, L.; Kishore, V.S.; Basha, S.N. Formulation and evaluation of poorly soluble febuxostat orodispersable tablet. AjaddCoUk 2014, 2, 191–202. [Google Scholar]
- Kuchekar, B.; Divekar, B.; Jagdale, S.; Gonjari, I. Solubility enhancement and formulation of rapid disintegrating tablet of febuxostat cyclodextrin complex. JPR Solut. 2013, 1, 168–175. [Google Scholar]
- Kalaydina, R.V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef] [Green Version]
- Lehtisalo, M.; Keskitalo, J.E.; Tornio, A.; Lapatto-Reiniluoto, O.; Deng, F.; Jaatinen, T.; Viinamäki, J.; Neuvonen, M.; Backman, J.T.; Niemi, M. Febuxostat, but not allopurinol, markedly raises the plasma concentrations of the breast cancer resistance protein substrate rosuvastatin. Clin. Transl. Sci. 2020. [Google Scholar] [CrossRef]
- Uzu, M.; Nonaka, M.; Miyano, K.; Sato, H.; Kurebayashi, N.; Yanagihara, K.; Sakurai, T.; Hisaka, A.; Uezono, Y. A novel strategy for treatment of cancer cachexia targeting xanthine oxidase in the brain. J. Pharmacol. Sci. 2019, 140, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Yamauchi, T.; Fujita, K.; Lee, S.; Ookura, M.; Kishi, S.; Urasaki, Y.; Yoshida, A.; Iwasaki, H.; Ueda, T. Controlling serum uric acid using febuxostat in cancer patients at risk of tumor lysis syndrome. Oncol. Lett. 2014, 8, 1523–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffrath, J.; Schmoll, H.J.; Voigt, W.; Muller, L.P.; Muller-Tidow, C.; Mueller, T. Efficacy of targeted drugs in germ cell cancer cell lines with differential cisplatin sensitivity. PLoS ONE 2017, 12, e0178930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, J.E. Febuxostat: A review of its use in the treatment of hyperuricaemia in patients with gout. Drugs 2015, 75, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; Alghaith, A.F.; et al. Piceatannol-loaded emulsomes exhibit enhanced cytostatic and apoptotic activities in colon cancer cells. Antioxidants 2020, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Choi, S.Y.; Choi, H.J.; Ryu, H.M.; Kim, Y.J.; Jung, H.Y.; Cho, J.H.; Kim, C.D.; Park, S.H.; Kwon, T.H.; et al. The emerging role of xanthine oxidase inhibition for suppression of breast cancer cell migration and metastasis associated with hypercholesterolemia. FASEB J. 2019, 33, 7301–7314. [Google Scholar] [CrossRef] [PubMed]
- Ucisik, M.H.; Kupcu, S.; Schuster, B.; Sleytr, U.B. Characterization of curcuemulsomes: Nanoformulation for enhanced solubility and delivery of curcumin. J. Nanobiotechnol. 2013, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Forrest, M.D. Why cancer cells have a more hyperpolarised mitochondrial membrane potential and emergent prospects for therapy. bioRxiv 2015, 025197. [Google Scholar]
- Houston, M.A.; Augenlicht, L.H.; Heerdt, B.G. Stable differences in intrinsic mitochondrial membrane potential of tumor cell subpopulations reflect phenotypic heterogeneity. Int. J. Cell Biol. 2011, 2011, 978583. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, A.; Mantuano, E.; Matarrese, P.; Saccomanni, G.; Manera, C.; Mattei, V.; Gambardella, L.; Malorni, W.; Sorice, M.; Misasi, R. A new 4-phenyl-1,8-naphthyridine derivative affects carcinoma cell proliferation by impairing cell cycle progression and inducing apoptosis. Anticancer Agents Med. Chem. 2012, 12, 653–662. [Google Scholar] [CrossRef]
- Song, I.S.; Kim, H.K.; Lee, S.R.; Jeong, S.H.; Kim, N.; Ko, K.S.; Rhee, B.D.; Han, J. Mitochondrial modulation decreases the bortezomib-resistance in multiple myeloma cells. Int. J. Cancer 2013, 133, 1357–1367. [Google Scholar] [CrossRef] [PubMed]








| Independent Variables | Levels | ||
| (−1) | (0) | (+1) | |
| X1: FBX concentration (% w/w) | 0.20 | 0.45 | 0.70 |
| X2: PC concentration (% w/w) | 1.00 | 2.50 | 4.00 |
| X3: Ultrasonication time (min) | 1.00 | 3.00 | 5.00 |
| Responses | Desirability Constraints | ||
| Y1: Particle size (nm) | Minimize | ||
| Experimental Run # | Independent Variables | Particle Size (nm) ± SD | ||
|---|---|---|---|---|
| FBX Concentration (% w/w) | PC Concentration (% w/w) | Ultrasonication Time (min) | ||
| F1 | 0.20 | 1.00 | 3.00 | 136.15 ± 2.89 |
| F2 | 0.70 | 4.00 | 3.00 | 170.48 ± 2.63 |
| F3 | 0.45 | 2.50 | 3.00 | 156.76 ± 1.67 |
| F4 | 0.20 | 2.50 | 5.00 | 90.70 ± 1.19 |
| F5 | 0.20 | 4.00 | 3.00 | 163.07 ± 2.34 |
| F6 | 0.20 | 2.50 | 1.00 | 183.80 ± 3.11 |
| F7 | 0.70 | 2.50 | 5.00 | 112.42 ± 1.93 |
| F8 | 0.45 | 4.00 | 1.00 | 200.17 ± 3.98 |
| F9 | 0.45 | 2.50 | 3.00 | 151.81 ± 2.11 |
| F10 | 0.45 | 4.00 | 5.00 | 129.80 ± 1.45 |
| F11 | 0.70 | 2.50 | 1.00 | 186.07 ± 2.47 |
| F12 | 0.70 | 1.00 | 3.00 | 145.82 ± 2.19 |
| F13 | 0.45 | 2.50 | 3.00 | 151.68 ± 1.72 |
| F14 | 0.45 | 1.00 | 1.00 | 180.32 ± 2.27 |
| F15 | 0.45 | 2.50 | 3.00 | 152.72 ± 1.99 |
| F16 | 0.45 | 2.50 | 3.00 | 155.13 ± 1.33 |
| F17 | 0.45 | 1.00 | 5.00 | 79.97 ± 0.98 |
| Responses | Model | Sequential p-Value | Lack of Fit p-Value | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | PRESS | Significant Terms |
|---|---|---|---|---|---|---|---|---|---|
| Y: Particle size (nm) | Quadratic | 0.0046 | 0.1420 | 0.9959 | 0.9907 | 0.9522 | 47.66 | 813.53 | X1, X2, X3, X1X3, X2X3, X32 |
| Variables | X1: FBX Concentration (% w/w) | X2: PC Concentration (% w/w) | X3: Ultrasonication Time (min) |
|---|---|---|---|
| Optimum values | 0.21 | 1.07 | 4.88 |
| Vesicle size (nm) | Predicted value | Observed value | Error % |
| 77.94 | 77.89 | 3.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmy, U.A.; Aldawsari, H.M.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Alhakamy, N.A.; Alsulimani, H.H.; Caraci, F.; Caruso, G. The Encapsulation of Febuxostat into Emulsomes Strongly Enhances the Cytotoxic Potential of the Drug on HCT 116 Colon Cancer Cells. Pharmaceutics 2020, 12, 956. https://doi.org/10.3390/pharmaceutics12100956
Fahmy UA, Aldawsari HM, Badr-Eldin SM, Ahmed OAA, Alhakamy NA, Alsulimani HH, Caraci F, Caruso G. The Encapsulation of Febuxostat into Emulsomes Strongly Enhances the Cytotoxic Potential of the Drug on HCT 116 Colon Cancer Cells. Pharmaceutics. 2020; 12(10):956. https://doi.org/10.3390/pharmaceutics12100956
Chicago/Turabian StyleFahmy, Usama A., Hibah M. Aldawsari, Shaimaa M. Badr-Eldin, Osama A. A. Ahmed, Nabil A. Alhakamy, Helal H. Alsulimani, Filippo Caraci, and Giuseppe Caruso. 2020. "The Encapsulation of Febuxostat into Emulsomes Strongly Enhances the Cytotoxic Potential of the Drug on HCT 116 Colon Cancer Cells" Pharmaceutics 12, no. 10: 956. https://doi.org/10.3390/pharmaceutics12100956