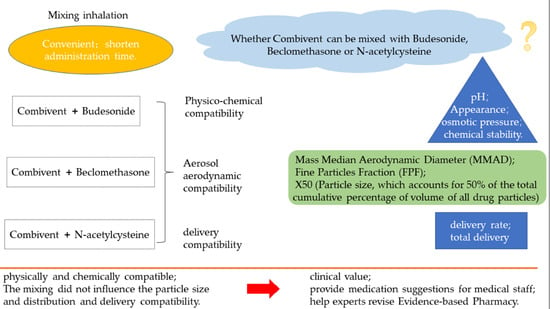
Aerosol Characteristics and Physico-Chemical Compatibility of Combivent® (Containing Salbutamol and Ipratropium Bromide) Mixed with Three Other Inhalants: Budesonide, Beclomethasone or N-Acetylcysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of Physico-Chemical Compatability
2.3. Measurement of Particle Size and Distribution
2.3.1. Cascade Impactor Method
2.3.2. Laser Diffraction Method
2.4. Delivery Rate and Total Delivery
3. Results
3.1. The Physico-Chemical Compataibility
3.2. Measurement of Particle Size and Distribution
3.2.1. Method of the Cascade Impactor
3.2.2. Laser Diffraction Method
3.3. Delivery Rate and Total Delivery
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Kruijf, W.; Ehrhardt, C. Inhalation delivery of complex drugs—The next steps. Curr. Opin. Pharmacol. 2017, 36, 52–57. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Pirozynski, M.; Sosnowski, T.R. Inhalation devices: From basic science to practical use, innovative vs. generic products. Expert Opin. Drug Deliv. 2016, 13, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.; Fink, J.B. Inhalation therapy in patients with tracheostomy: A guide to clinicians. Expert Rev. Respir. Med. 2017, 11, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Adorni, G.; Seifert, G.; Buttini, F.; Colombo, G.; Stecanella, L.A.; Krämer, I.; Rossi, A. Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects. Pharmaceutics 2019, 11, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, J.N. Nebulized drug delivery in respiratory medicine: What does the future hold? Ther. Deliv. 2017, 8, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, J.; Zhan, S.; Zhang, R.; Tan, W. Effects of Temperature and Humidity on Laser Diffraction Measurements to Jet Nebulizer and Comparison with NGI. AAPS PharmSciTech 2016, 17, 380–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.S. Pulmonary drug delivery system: Newer patents. Pharm. Pat. Anal. 2017, 6, 225–244. [Google Scholar] [CrossRef]
- Kamin, W.; Schwabe, A.; Krämer, I. Inhalation solutions: Which one are allowed to be mixed? Physico-Chemical compatibility of drug solutions in nebulizers. J. Cyst. Fibros. 2006, 5, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Burchett, D.K.; Darko, W.; Zahra, J.; Noviasky, J.; Probst, L.; Smith, A. Mixing and compatibility guide for commonly used aerosolized medications. Am. J. Health Syst. Pharm. 2010, 67, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Itazawa, T.; Adachi, Y.; Ito, Y.; Higuchi, O.; Mochizuki, H.; Shimojo, N.; Inoue, T. Aerosol Characteristics of Admixture of Budesonide Inhalation Suspension with a Beta2-Agonist, Procaterol. Allergol. Int. 2013, 62, 131–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonasia, P.J.; Mcvicar, W.K.; Williams, B.; Ong, S. Chemical and physical compatibility of levalbuterol inhalation solution concentrate mixed with budesonide, ipratropium bromide, cromolyn sodium, or acetylcysteine sodium. Respir. Care 2008, 53, 1716–1722. [Google Scholar] [PubMed]
- Bonasia, P.; Cook, C.; Cheng, Y.; Ong, S. Compatibility of arformoterol tartrate inhalation solution with three nebulized drugs. Curr. Med. Res. Opin. 2007, 23, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Kamin, W.; Schwabe, A.; Krämer, I. Physicochemical compatibility of fluticasone-17-Propionate nebulizer suspension with ipratropium and albuterol nebulizer solutions. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 599–607. [Google Scholar] [PubMed]
- Kramer, I.; Schwabe, A.; Lichtinghagen, R.; Kamin, W. Physicochemical compatibility of nebulizable drug mixtures containing dornase alfa and ipratropium and/or albuterol. Pharmazie 2007, 62, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Routine nebulized ipratropium and albuterol together are better than either alone in COPD. The COMBIVENT Inhalation Solution Study Group. Chest 1997, 112, 1514–1521. [Google Scholar] [CrossRef]
- Saito, M.; Kikuchi, Y.; Kawarai Lefor, A.; Hoshina, M. High-Dose nebulized budesonide is effective for mild asthma exacerbations in children under 3 years of age. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 22–27. [Google Scholar]
- Terzano, C.; Ricci, A.; Burinschi, V.; Nekam, K. Comparison of the efficacy of beclometasone dipropionate and fluticasone propionate suspensions for nebulization in adult patients with persistent asthma. Respir. Med. 2003, 97, S35–S40. [Google Scholar] [CrossRef]
- Patel, A.; Theokli, C. Nebulised N-Acetylcysteine used in acute tracheostoma obstruction. J. Laryngol. Otol. 2014, 128, 1123–1124. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, R.; Beng, H.; Deng, L.; Ke, Q.; Tan, W. Effects of flow pattern, device and formulation on particle size distribution of nebulized aerosol. Int. J. Pharm. 2019, 560, 35–46. [Google Scholar] [CrossRef]
- Mccallion, O.N.; Taylor, K.M.; Thomas, M.; Taylor, A.J. Nebulization of fluids of different physicochemical properties with air-jet and ultrasonic nebulizers. Pharm. Res. 1995, 12, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chen, C.M.; Lee, C.N.; Chiang, Y.C.; Chen, H.Y. Compatibility and osmolality of inhaled N-Acetylcysteine nebulizing solution with fenoterol and ipratropium. Am. J. Health Syst. Pharm. 2005, 62, 828–833. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Cruz-Rivera, M. Compatibility of budesonide inhalation suspension with four nebulizing solutions. Ann. Pharm. 2004, 38, 967–972. [Google Scholar] [CrossRef] [PubMed]



| Time (min) | Flow Rate (mL/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 1.00 | 95.0 | 5.0 |
| 10.00 | 1.00 | 80.0 | 20.0 |
| 15.00 | 1.00 | 80.0 | 20.0 |
| 16.00 | 1.00 | 95.0 | 5.0 |
| 25.00 | 1.00 | 95.0 | 5.0 |
| Time (min) | Flow Rate (mL/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 1.00 | 95.0 | 5.0 |
| 10.00 | 1.00 | 80.0 | 20.0 |
| 12.00 | 1.00 | 80.0 | 20.0 |
| 12.10 | 1.00 | 30.0 | 70.0 |
| 20.00 | 1.00 | 30.0 | 70.0 |
| 20.10 | 1.00 | 95.0 | 5.0 |
| 25.00 | 1.00 | 95.0 | 5.0 |
| Admixture Study | pH of Inhalation Solution/Suspension Prior to Admixing | pH of Admixture | 30 min vs. 0 min (%) | 24 h vs. 0 min (%) | |||
|---|---|---|---|---|---|---|---|
| Combivent® | Other Inhalation Solutions/Suspensions | 0 min | 30 min | 24 h | |||
| Combivent® + Pulmicort® | 3.55 ± 0.02 | 4.48 ± 0.06 | 4.21 ± 0.00 | 4.25 ± 0.02 | 4.20 ± 0.03 | 0.95 | 0.32 |
| Combivent® + Clenil® | 3.55 ± 0.02 | 6.26 ± 0.03 | 3.73 ± 0.04 | 3.73 ± 0.06 | 3.80 ± 0.03 | 0.18 | 1.97 |
| Combivent® + Fluimucil® | 3.55 ± 0.02 | 6.62 ± 0.01 | 6.52 ± 0.01 | 6.49 ± 0.02 | 6.37 ± 0.03 | 0.41 | 2.25 |
| Admixture Study | Osmotic Pressure of Inhalation Solution/Suspension Prior to Admixing (mOsm/kg) | Osmotic Pressure of Admixture (mOsm/kg) | 30 min vs. 0 min (%) | 24 h vs. 0 min (%) | |||
|---|---|---|---|---|---|---|---|
| Combivent® | Other Inhalation Solutions/Suspensions | 0 min | 30 min | 24 h | |||
| Combivent® + Pulmicort® | 276.67 ± 0.47 | 270.33 ± 0.47 | 282.67 ± 0.94 | 284.00 ± 2.16 | 278.00 ± 0.00 | 0.47 | 1.65 |
| Combivent® + Clenil® | 276.67 ± 0.47 | 283.00 ± 0.82 | 290.00 ± 1.63 | 287.33 ± 0.94 | 289.67 ± 0.94 | 0.92 | 0.11 |
| Combivent® + Fluimucil® | 276.67 ± 0.47 | 1312.67 ± 2.05 | 813.67 ± 2.87 | 818.33 ± 0.47 | 807.33 ± 1.89 | 0.57 | 0.78 |
| Admixture Study | Active Ingredients | 0 min Single vs. Admixture (%) | 30 min Single vs. Admixture (%) | 24 h Single vs. Admixture (%) | 30 min vs. 0 min (%) | 24 h vs. 0 min (%) |
|---|---|---|---|---|---|---|
| Combivent® + Pulmicort® | Ipratropium | 1.7 | 0.4 | 3.6 | 1.0 | 1.7 |
| Salbutamol | 5.9 | 4.1 | 7.6 | 1.2 | 0.9 | |
| Budesonide | 5.9 | 9.4 | 9.5 | 2.4 | 4.7 | |
| Combivent® + Clenil® | Ipratropium | 1.8 | 2.9 | 6.3 | 1.5 | 0.8 |
| Salbutamol | 2.6 | 3.7 | 6.5 | 1.7 | 1.3 | |
| Beclomethasone | 3.3 | 2.8 | 7.1 | 3.0 | 1.3 | |
| Combivent® + Fluimucil® | Ipratropium | 6.7 | 6.9 | 2.0 | 0.0 | 8.7 |
| Salbutamol | 1.0 | 1.4 | 1.7 | 0.0 | 2.2 | |
| N-Acetylcysteine | 6.9 | 6.0 | 3.3 | 0.0 | 0.4 |
| Admixture Study | Active Ingredients | MMAD (µm) | FPF (%) | MMAD Change Percentage (%) | FPF Change Percentage (%) |
|---|---|---|---|---|---|
| Combivent® | Ipratropium | 5.01 ± 0.05 | 48.64 ± 0.45 | ||
| Salbutamol | 4.98 ± 0.07 | 48.86 ± 0.56 | |||
| Pulmicort® | Budesonide | 6.54 ± 0.22 | 32.10 ± 2.32 | ||
| Clenil® | Beclomethasone | 6.96 ± 0.02 | 27.16 ± 0.26 | ||
| Fluimucil® | N-Acetylcysteine | 4.94 ± 0.07 | 49.98 ± 0.71 | ||
| Combivent® + Pulmicort® | Ipratropium | 4.90 ± 0.08 | 49.59 ± 0.78 | 2.21 | 1.92 |
| Salbutamol | 5.40 ± 0.03 | 45.33 ± 0.27 | 2.63 | 2.42 | |
| Budesonide | 6.25 ± 0.17 | 34.99 ± 1.56 | 4.57 | 8.26 | |
| Combivent® + Clenil® | Ipratropium | 5.45 ± 0.03 | 45.00 ± 0.37 | 8.07 | 8.09 |
| Salbutamol | 5.40 ± 0.03 | 45.33 ± 0.27 | 7.88 | 7.80 | |
| Beclomethasone | 6.99 ± 0.20 | 27.48 ± 1.55 | 0.39 | 1.19 | |
| Combivent® + Fluimucil® | Ipratropium | 4.70 ± 0.05 | 51.87 ± 0.64 | 6.50 | 6.23 |
| Salbutamol | 4.85 ± 0.09 | 50.07 ± 0.87 | 7.03 | 6.71 | |
| N-Acetylcysteine | 4.78 ± 0.08 | 51.71 ± 0.86 | 3.35 | 3.34 |
| Admixture Study | X10 (µm) | X50 (µm) | X90 (µm) | Span |
|---|---|---|---|---|
| Combivent® | 0.72 ± 0.04 | 2.54 ± 0.22 | 8.38 ± 0.41 | 3.03 ± 0.11 |
| Pulmicort® | 0.69 ± 0.02 | 2.66 ± 0.08 | 8.96 ± 0.01 | 3.12 ± 0.10 |
| Clenil® | 0.78 ± 0.01 | 2.88 ± 0.04 | 8.96 ± 0.07 | 2.84 ± 0.02 |
| Fluimucil® | 0.65 ± 0.04 | 2.51 ± 0.03 | 8.26 ± 0.16 | 3.03 ± 0.03 |
| Combivent® + Pulmicort® | 0.82 ± 0.11 | 3.20 ± 0.01 | 9.85 ± 0.24 | 2.82 ± 0.04 |
| Combivent® + Clenil® | 0.76 ± 0.01 | 3.27 ± 0.09 | 9.87 ± 0.16 | 2.79 ± 0.05 |
| Combivent® + Fluimucil® | 0.86 ± 0.01 | 2.91 ± 0.03 | 8.77 ± 0.04 | 2.72 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Hu, J.; Deng, L.; Li, S.; Chen, X.; Liu, F.; Wang, S.; Mohammed Abdul, K.S.; Beng, H.; Tan, W. Aerosol Characteristics and Physico-Chemical Compatibility of Combivent® (Containing Salbutamol and Ipratropium Bromide) Mixed with Three Other Inhalants: Budesonide, Beclomethasone or N-Acetylcysteine. Pharmaceutics 2020, 12, 78. https://doi.org/10.3390/pharmaceutics12010078
Zhang R, Hu J, Deng L, Li S, Chen X, Liu F, Wang S, Mohammed Abdul KS, Beng H, Tan W. Aerosol Characteristics and Physico-Chemical Compatibility of Combivent® (Containing Salbutamol and Ipratropium Bromide) Mixed with Three Other Inhalants: Budesonide, Beclomethasone or N-Acetylcysteine. Pharmaceutics. 2020; 12(1):78. https://doi.org/10.3390/pharmaceutics12010078
Chicago/Turabian StyleZhang, Rui, Junhua Hu, Liangjun Deng, Sha Li, Xi Chen, Fei Liu, Shanping Wang, Khaja Shameem Mohammed Abdul, Huimin Beng, and Wen Tan. 2020. "Aerosol Characteristics and Physico-Chemical Compatibility of Combivent® (Containing Salbutamol and Ipratropium Bromide) Mixed with Three Other Inhalants: Budesonide, Beclomethasone or N-Acetylcysteine" Pharmaceutics 12, no. 1: 78. https://doi.org/10.3390/pharmaceutics12010078