Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations
Abstract
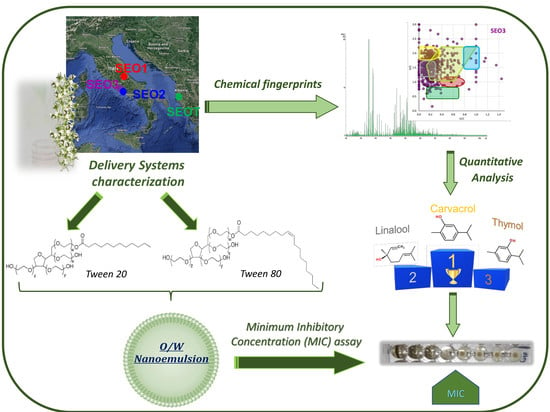
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Electrospray Ionization FT-ICR MS Analysis
2.3. Gas Chromatography/Mass Spectrometry Analysis
2.4. Nanoemulsion Preparation
2.5. Dynamic Light Scattering Measurements
2.6. Transmission Electron Microscopy (TEM)
2.7. NEs Stability Evaluation
2.8. Fluorometric Measurements
2.9. In Vitro Release Study
2.10. Bacterial Strains
2.11. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
2.12. Determination of the Effect of SEOs and NEs on T24 Cell by MTT Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. MS Metabolite Profiling
3.2. GC-MS Analysis of the Volatile Components
3.3. Characterization of Satureja Montana L. Essential Oil Nanoemulsions
3.4. Antimicrobial Susceptibility Assays
3.5. Antibacterial Activity of EOs and NEs
3.6. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fatemeh, J.; Zahra, L.; Hossein, A. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Asprea, M.; Risaliti, L.; Vanti, G.; Bergonzi, M.C. Plants extracts loaded in nanocarriers: An emergent formulating approach. Nat. Prod. Commun. 2018, 13, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A critical overview on the pharmacological and clinical aspects of popular satureja species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef]
- Milos, M.; Radonic, A.; Bezic, N.; Dunkic, V. Localities and seasonal variations in the chemical composition of essential oils of Satureja montana L. and S. cuneifolia Ten. Flavour Frag. J. 2001, 16, 157–160. [Google Scholar] [CrossRef]
- Kremer, D.; Košir, I.J.; Končić, M.Z.; Čerenak, A.; Potočnik, T.; Srečec, S.; Randić, M.; Kosalec, I. Antimicrobial and Antioxidant Properties of Satureja montana L. and S. subspicata Vis. (Lamiaceae). Curr. Drug Targets 2015, 16, 1623–1633. [Google Scholar] [CrossRef]
- Elgndi, M.A.; Filip, S.; Pavlić, B.; Vladić, J.; Stanojković, T.; Žižak, Ž.; Zeković, Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 128–137. [Google Scholar] [CrossRef]
- Cavar, S.; Maksimovic, M.; Solic, M.E.; Jerkovic-Mujkic, A.; Besta, R. Chemical composition and antioxidant and antimicrobial activity of two Satureja essential oils. Food Chem. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Momtaz, S.; Abdollahi, M. An update on pharmacology of Satureja species; From antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. Int. J. Pharmacol. 2010, 6, 454–461. [Google Scholar] [CrossRef]
- Souto-Maior, F.N.; da Fonsêca, D.V.; Rodrigues Salgado, P.R.; de Oliveira Monte, L.; de Sousa, D.P.; de Almeida, R.N. Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm. Biol. 2017, 55, 63–67. [Google Scholar] [CrossRef]
- Kustrak, D.; Kuftinec, J.; Blazevic, N.; Maffei, M. Comparison of the essential oil composition of two subspecies of Satureja montana. J. Essent. Oil Res. 1996, 8, 7–13. [Google Scholar] [CrossRef]
- Hazem, S.E.; Ippolito, C. An overview of the biological effects of some mediterranean essential oils on human health. BioMed. Res. Int. 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Baydar, H.; Sagdic, O.; Ozkan, G.; Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Šućur, J.; Popović, A.; Petrović, M.; Anačkov, G.; Bursić, V.; Kiprovski, B.; Prvulović, D. Allelopathic effects and insecticidal activity of aqueous extracts of Satureja montana L. J. Serb. Chem. Soc. 2015, 80, 125–128. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Slavkovska, V.; Jancic, R. Variability of the essential oil composition of the species Satureja montana L. (Lamiaceae). J. Essent. Oil Res. 1997, 9, 629–634. [Google Scholar] [CrossRef]
- Chrpová, D.; Kourimska, L.; Gordon, M.H.; Heřmanová, V.; Roubíčková, I.; Pánek, J. Antioxidant activity of selected phenols and herbs used in diets for medical conditions. Czech J. Food Sci. 2010, 28, 317–335. [Google Scholar] [CrossRef] [Green Version]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef] [Green Version]
- Hajdari, A.; Mustafa, B.; Kaçiku, A.; Mala, X.; Lukas, B.; Ibraliu, A.; Stefkov, G.; Novak, J. Chemical composition of the essential oil, total phenolics, total flavonoids and antioxidant activity of methanolic extracts of Satureja montana L. Rec. Nat. Prod. 2016, 10, 750–760. [Google Scholar]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Aziz, M.M.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential—A review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F.; et al. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, R.; Athinarayanan, J.; Periasamy, V.S.; Adisa, A.R.; Al-Shuniaber, M.A.; Gassem, M.A.; Alshatw, A.A. Antimicrobial activity of nanoemulsion on drug-resistant bacterial pathogens. Microb. Pathog. 2018, 120, 85–96. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Witting, M.; Moritz, F.; Gil, R.B.; Goffette, D.; Valade, M.; Schmitt-Kopplin, P.; Gougeon, R.D. Natural oxygenation of Champagne wine during ageing on lees: A metabolomics picture of hormesis. Food Chem. 2016, 203, 207–215. [Google Scholar] [CrossRef]
- Maia, M.; Monteiro, F.; Sebastiana, M.; Marques, A.P.; Ferreira, A.E.N.; Freire, A.P.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Metabolite extraction for high-throughput FTICR-MS-based metabolomics of grapevine leaves. EuPA Open Proteom. 2016, 12, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Wägele, B.; Witting, M.; Suhre, K. MassTRIX reloaded: Combined analysis and visualization of transcriptome and metabolome data. PLoS ONE 2012, 7, e39860. [Google Scholar] [CrossRef] [Green Version]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Siuzdak, G. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [Green Version]
- Patiny, L.; Borel, A. ChemCalc: A building block for tomorrow’s chemical infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hanieh, P.N.; Longhi, C.; Carradori, S.; Secci, D.; Zengin, G.; Ammendolia, M.G.; Mattia, E.; Del Favero, E.; Marianecci, C.; et al. Neem Oil Nanoemulsions: Characterisation and antioxidant activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 1265–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachariasse, K.A. Intramolecular excimer formation with diarylalkanes as a microfluidity probe for sodium dodecyl sulphate micelles. Chem. Phys. Lett. 1978, 57, 429–432. [Google Scholar] [CrossRef]
- Ingallina, C.; Rinaldi, F.; Bogni, A.; Ponti, J.; Passeri, D.; Reggente, M.; Rossi, M.; Kinsner-Ovas Kainem, A.; Mehn, D.; Rossi, F.; et al. Niosomal approach to brain delivery: Development, characterization and in vitro toxicological studies. Int. J. Pharm. 2016, 511, 969–982. [Google Scholar] [CrossRef]
- Rinaldi, F.; Del Favero, E.; Moeller, J.; Hanieh, P.N.; Passeri, D.; Rossi, M.; Angeloni, L.; Venditti, I.; Marianecci, C.; Carafa, M.; et al. Hydrophilic silver nanoparticles loaded into niosomes: Physical–chemical characterization in view of biological applications. Nanomater. Basel 2019, 9, 1177. [Google Scholar] [CrossRef] [Green Version]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Becker, L.; Poutaraud, A.; Hamm, J.; Muller, J.F.; Merdinoglu, D.; Carré, V.; Chaimbault, P. Metabolic study of grapevine leaves infected by downy mildew using negative ion electrospray—Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chim. Acta 2013, 795, 44–51. [Google Scholar] [CrossRef]
- Kirwan, J.A.; Weber, R.J.; Broadhurst, D.I.; Viant, M.R. Direct infusion mass spectrometry metabolomics dataset: A benchmark for data processing and quality control. Sci. Data 2014, 1, 140012. [Google Scholar] [CrossRef] [Green Version]
- Pascale, R.; Bianco, G.; Cataldi, T.R.I.; Schmitt-Kopplin, P.; Bosco, F.; Vignola, L.; Uhl, J.; Lucio, M.; Milella, L. Mass spectrometry-based phytochemical screening for hypoglycemic activity of Fagioli di Sarconi beans (Phaseolus vulgaris L.). Food Chem. 2018, 242, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, A.P.; Mannina, L.; Capitani, D.; Sanzò, G.; Ingallina, C.; Botta, B.; Fornarini, S.; Crestoni, M.E.; Chiavarino, B.; Carradori, S.; et al. A multi-methodological approach in the study of Italyn PDO “Cornetto di Pontecorvo” red sweetpepper. Food Chem. 2018, 255, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Cioni, P.; Morelli, I. Essential oil of Satureja montana L. ssp. Montana. Composition and Yields of Plants Grown Under different environmental conditions. J. Essent. Oil Res. 1992, 4, 563–568. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of essential oils obtained from abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Souto-Maior, F.N.; de Carvalho, F.L.; de Morais, L.C.; Netto, S.M.; de Sousa, D.P.; de Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.J.; Alonso, A.; Garcìa-Martìnez, S.; Valero, M.; Blasco, P.; Ruiz-Bevia, F. Quantitative analysis of flavour volatiles detects differences among closely related traditional cultivars of tomato. J. Sci. Food Agric. 2005, 85, 54–60. [Google Scholar] [CrossRef]
- Rùa, J.; del Valle, P.; de Arriaga, D.; Fernández-Álavarez, L.; García-Armesto, M.R. Combination of carvacrol and thymol: Antimicrobial activity against Staphylococcus aureus and antioxidant activity. Food. Path. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- Labhasetwar, V.; Mohan, M.S.; Dorle, A.K. A study on zeta potential and dielectric constant of liposomes. J. Microencapsul. 1994, 11, 663–668. [Google Scholar] [CrossRef]
- Tsermentseli, S.K.; Kontogiannopoulos, K.N.; Papageorgiou, V.P.; Assimopoulou, V.P. Comparative study of PEGylated and conventional liposomes as carriers for shikonin. Fluids 2018, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Stachurski, J.; MichaŁek, M. The Effect of the ζ potential on the stability of a non-polar oil-in-water emulsion. J. Colloid Interface Sci. 1996, 184, 433–436. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Roger, K.; Cabane, B. Why are hydrophobic/water interfaces negatively charged? Angew. Chem. Int. Ed. 2012, 51, 5625–5628. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R.; Biniaz, M. Toxicity of essential oil of Satureja khuzistanica: In vitro cytotoxicity and anti-microbial activity. J. Immunotoxicol. 2013, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Javad Sharifi-Rad, J.; Sharifi-Rad, M.; Hoseini-Alfatemi, S.M.; Iriti, M.; Majid Sharifi-Rad, M.; Sharifi-Rad, M. Composition, cytotoxic and antimicrobial activities of satureja intermedia C.A. mey essential oil. Int. J. Mol. Sci. 2015, 16, 17812–17825. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, I.; Yousefzadi, M.; Behmanesh, M.; Sharifi, M.; Moradi, A. In vitro cytotoxic and antimicrobial activity of essential oil from satureja intermedia. Iran. Red. Crescent Med. J. 2013, 15, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.R.F.; Schuh, R.S.; Jacques, A.L.B.; Augustin, O.A.; Bordignon, S.A.L.; Dias, D.O.; Kelmann, R.G.; Koester, L.S.; Gehring, M.P.; Morrone, F.B.; et al. Cytotoxic activity evaluation of essential oils and nanoemulsions of Drimys angustifolia and D. brasiliensis on human glioblastoma (U-138 MG) and human bladder carcinoma (T24) cell lines in vitro. Rev. Bras. Farmacogn. 2013, 23, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Kundaković, T.; Stanojković, T.; Kolundzija, B.; Marković, S.; Sukilović, B.; Milenković, M.; Lakusić, B. Cytotoxicity and antimicrobial activity of the essential oil from Satureja montana subsp. pisidica (Lamiaceae). Nat. Prod. Commun. 2014, 9, 569–572. [Google Scholar]
- Liang, W.Z.; Lu, C.H. Carvacrol-induced [Ca2+] i rise and apoptosis in human glioblastoma cells. Life Sci. 2012, 90, 703–711. [Google Scholar] [CrossRef]
- Hsu, S.S.; Lin, K.L.; Chou, C.T.; Chiang, A.J.; Liang, W.Z.; Chang, H.T.; Tsai, J.Y.; Liao, W.C.; Huang, F.D.; Huang, J.K.; et al. Effect of thymol on Ca2+ homeostasis and viability in human glioblastoma cells. Eur. J. Pharmacol. 2011, 670, 85–91. [Google Scholar] [CrossRef]
- Tzou Chi Huang, T.; Ting Lin, Y.; Pin Chuang, K. Carvacrol has the priming effects of reactive oxygen species (ROS) production in C6 glioma cells. Food Agric. Immunol. 2010, 21, 47–55. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R. Non-nitrogenous Plant-derived Constituents with Antiplasmodial Activity. Nat. Prod. Commun. 2006, 1, 1181–1204. [Google Scholar] [CrossRef]










| Samples | SEO3 [a] | Tw20 [a] | Tw80 [a] |
|---|---|---|---|
| A1 | 50 | 50 | |
| A2 | 33.3 | 66.6 | |
| B1 | 50 | 50 | |
| B2 | 33.3 | 66.6 |
| Class | Compound | SEO1 | SEO2 | SEO3 | SEOT (a) |
|---|---|---|---|---|---|
| Monoterpene | α-thujene | - | - | - | 0.1 |
| α-pinene | 0.4 | 0.4 | 0.4 | 1.3 | |
| camphene | 0.1 | 0.1 | 0.2 | 0.4 | |
| β-pinene | 0.2 | 0.2 | 0.2 | - | |
| β-myrcene | 0.1 | 0.1 | 0.5 | 0.6 | |
| 3-carene | - | - | - | 0.2 | |
| α-terpinene | - | - | - | 0.8 | |
| p-cymene | 10.3 | 9.4 | 12.3 | 15.3 | |
| limonene | 1.3 | 1.3 | 1.8 | 0.9 | |
| Terpenoid | eucalyptol | 6.2 | 6.3 | 6.8 | - |
| Monoterpene | ocimene | - | - | 0.2 | - |
| γ-terpinene | 12.3 | 14.7 | 15.0 | 4.5 | |
| Terpenoid | linaloxide (cis) | 0.4 | 0.2 | 0.2 | - |
| Monoterpene | terpinolene | - | - | - | 0.1 |
| Terpenoid | linaloxide (trans) | 0.3 | 0.2 | 0.1 | - |
| linalool | 16.1 | 16.0 | 16.4 | - | |
| camphor | 0.2 | 0.2 | 0.3 | 0.1 | |
| borneol | - | - | 0.5 | 3.1 | |
| terpinen-4-ol | - | - | - | 1.2 | |
| γ-terpineol | - | - | 0.2 | - | |
| α-terpineol | 1.5 | 1.5 | 1.6 | - | |
| thymolmethylether | - | - | - | 4.3 | |
| D-carvone | 1.6 | 1.5 | 1.6 | - | |
| thymol | 16.5 | 16.1 | 14.5 | 7.6 | |
| carvacrol | 29.0 | 28.7 | 23.9 | 43.9 | |
| eugenol | 1.0 | 0.8 | 0.4 | - | |
| Sesquiterpene | β-caryophyllene | 0.1 | 0.2 | 0.4 | 3.4 |
| α-humulene | - | - | 0.1 | - | |
| caryophylleneoxie | 0.4 | 0.2 | - | - | |
| unidentified | 2.0 | 1.9 | 2.4 | 12.2 | |
| Monoterpene | 24.7 | 26.2 | 30.6 | 24.2 | |
| Terpenoid | 72.8 | 71.5 | 66.5 | 60.2 | |
| Sesquiterpene | 0.5 | 0.4 | 0.5 | 3.4 |
| Compound | Mass Percentage |
|---|---|
| carvacrol | 29.9 |
| thymol | 16.0 |
| Samples | HD (nm) ± SD | ζ-Pot (mV) ± SD | PDI ± SD |
|---|---|---|---|
| A1 | 118.40 ± 0.61 | −19.00 ± 0.21 | 0.26 ± 0.01 |
| A2 | 55.86 ± 0.93 | −16.61 ± 1.06 | 0.18 ± 0.03 |
| B1 | 95.05 ± 1.55 | −13.02 ± 0.36 | 0.23 ± 0.01 |
| B2 | 56.08 ± 1.17 | −14.14 ± 0.25 | 0.17 ± 0.01 |
| Sample | IE/I3 (Microviscosity) ± SD | I1/I3 (Polarity) ± SD |
|---|---|---|
| A1 | 4.49 ± 0.07 | 1.62 ± 0.13 |
| A2 | 4.04 ± 0.16 | 1.47 ± 0.23 |
| B1 | 4.69 ± 0.11 | 1.44 ± 0.07 |
| B2 | 3.79 ± 0.21 | 1.50 ± 0.06 |
| Bacterial Strain | Antibiotic Resistance Profile |
|---|---|
| L. monocytogenes | Oxacillin, Levofloxacin, Daptomycin |
| S. aureus (SA1) | Erythromycin, Fosfomycin, Gentamicin, Levofloxacin, Oxacillin, Rifampicin, Tetracycline, Sulfamethoxazole/Trimethoprim |
| S. aureus (SA2) | Benzylpenicillin, Clindamycin, Erythromycin, Levofloxacin, Oxacillin |
| S. haemolyticus | Clindamycin, Erythromycin, Fosfomycin, Gentamicin, Levofloxacin, Oxacillin, Sulfamethoxazole/Trimethoprim |
| E. coli | Full sensitive |
| K. pneumoniae | Amoxicillin/Clavulanate, Cefepime, Cefotaxime, Ceftazidime, Ciprofloxacin, Fosfomycin, Gentamicin, Sulfamethoxazole/Trimethoprim |
| P. aeruginosa | Amoxicillin/Clavulanate, Cefotaxime, Ertapenem, Fosfomycin, Tigecycline, Sulfamethoxazole/Trimethoprim |
| S. marcescens | Amoxicillin/Clavulanate, Colistin |
| SEO1 | SEO2 | SEO3 | SEOT | |||||
|---|---|---|---|---|---|---|---|---|
| Bacterial Strain | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| L. monocytogenes | 1.56 | 3.12 | 1.56 | 3.12 | 0.78 | 1.56 | 0.78 | 0.78 |
| S. aureus (SA1) | 3.12 | 3.12 | 1.56 | 3.12 | 1.56 | 1.56 | 0.78 | 0.78 |
| S. aureus (SA2) | 3.12 | 3.12 | 1.56 | 1.56 | 1.56 | 1.56 | 0.78 | 0.78 |
| S. haemolyticus | 3.12 | 3.12 | 1.56 | 1.56 | 1.56 | 1.56 | 0.78 | 0.78 |
| E. coli | 3.12 | 6.25 | 1.56 | 3.12 | 3.12 | 3.12 | 1.56 | 1.56 |
| K. pneumoniae | 1.56 | 3.12 | 1.56 | 3.12 | 0.78 | 0.78 | 0.39 | 0.39 |
| P. aeruginosa | 3.12 | 3.12 | 3.12 | 3.12 | 3.12 | 3.12 | 1.56 | 1.56 |
| S. marcescens | 3.12 | 3.12 | 1.56 | 1.56 | 0.78 | 0.78 | 0.39 | 0.39 |
| Bacterial Strain | NE-A1 | NE-A2 | NE-B1 | NE-B2 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| L. monocytogenes | 0.78 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 |
| S. aureus (SA1) | 0.78 | 1.56 | 0.78 | 1.56 | 0.78 | 1.56 | 1.56 | 1.56 |
| S. aureus (SA2) | 0.78 | 1.56 | 0.78 | 1.56 | 0.78 | 1.56 | 1.56 | 1.56 |
| S. haemolyticus | 1.56 | 2.3 | 9.2 | 9.2 | 2.3 | 4.6 | 9.2 | 9.2 |
| E. coli | 0.39 | 0.78 | 1.56 | 1.56 | 0.39 | 1.56 | 1.56 | 1.56 |
| K. pneumoniae | 0.78 | 0.78 | 1.56 | 1.56 | 0.78 | 0.78 | 1.56 | 1.56 |
| P. aeruginosa | 4.6 | 4.6 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 |
| S. marcescens | 0.78 | 0.78 | 0.78 | 1.56 | 0.39 | 0.78 | 1.56 | 1.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations. Pharmaceutics 2020, 12, 7. https://doi.org/10.3390/pharmaceutics12010007
Maccelli A, Vitanza L, Imbriano A, Fraschetti C, Filippi A, Goldoni P, Maurizi L, Ammendolia MG, Crestoni ME, Fornarini S, et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations. Pharmaceutics. 2020; 12(1):7. https://doi.org/10.3390/pharmaceutics12010007
Chicago/Turabian StyleMaccelli, Alessandro, Luca Vitanza, Anna Imbriano, Caterina Fraschetti, Antonello Filippi, Paola Goldoni, Linda Maurizi, Maria Grazia Ammendolia, Maria Elisa Crestoni, Simonetta Fornarini, and et al. 2020. "Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations" Pharmaceutics 12, no. 1: 7. https://doi.org/10.3390/pharmaceutics12010007