Energy Transfer in Vials Nested in a Rack System During Lyophilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment and Materials
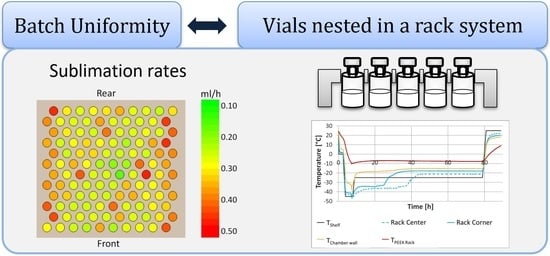
2.2. Vial Holding Systems
2.3. Excipients
2.4. Determination of Glass Transition Temperature Tg’ and Collapse Temperature Tc
2.5. Freeze Drying Procedure
2.6. Determination of Sublimation Rates
2.7. Modes of Energy Transfer and Impact of the Rack
3. Results and Discussion
3.1. Characterization of the Solution
3.2. Behavior of the Rack during Rreeze Drying
3.3. Modes of Energy Transfer in Separated Vials
3.4. Energy and Mass Transfer in a Rack System
3.5. Comparison of the Rack System to Another Nested Vial System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patient-centered drug manufacture. Nat. Biotechnol. 2017, 35, 485. [CrossRef] [PubMed] [Green Version]
- Pharma R&D Annual Review 2017. Available online: https://pharmaintelligence.informa.com/~/media/Informa-Shop-Window/Pharma/Files/PDFs/whitepapers/RD-Review-2017.pdf (accessed on 2 October 2019).
- Medicines in Development: Immuno-Oncology. Available online: http://phrma-docs.phrma.org/files/dmfile/GoBoldlyImmuno_OncologyReport_2017.pdf (accessed on 2 October 2019).
- Shanley, A. Packaging’s Flexible, Patient-Centered Future. BioPharm. International. 2017, 30, 48–50. [Google Scholar]
- Deutschle, G.; Selch, J. Nested vials for improved lyophilization efficiency. Int. Pharm. Ind. 2015, 7, 124–128. [Google Scholar]
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rambhatla, S.; Pikal, M.J. Heat and mass transfer scale-up issues during freeze-drying, I: Atypical radiation and the edge vial effect. AAPS PharmSciTech 2003, 4, E14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikal, M.J.; Roy, M.L.; Shah, S. Mass and Heat Transfer in Vial Freeze-Drying of Pharmaceuticals: Role of the Vial. J. Pharm. Sci. 1984, 73, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Brülls, M.; Rasmuson, A. Heat transfer in vial lyophilization. Int. J. Pharm. 2002, 246, 1–16. [Google Scholar] [CrossRef]
- Deutschle, G. AdaptiQ-Ready-to-Use Vials for Aseptic Processing; Schott AG: Mainz, Germany, 2016; pp. 1–20. [Google Scholar]
- Nail, S.; Tchessalov, S.; Shalaev, E.; Ganguly, A.; Renzi, E.; Dimarco, F.; Wegiel, L.; Ferris, S.; Kessler, W.; Pikal, M.; et al. Recommended Best Practices for Process Monitoring Instrumentation in Pharmaceutical Freeze Drying-2017. AAPS PharmSciTech 2017, 18, 2379–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegiel, L.A.; Ferris, S.J.; Nail, S.L. Experimental Aspects of Measuring the Vial Heat Transfer Coefficient in Pharmaceutical Freeze-Drying. AAPS PharmSciTech 2018, 19, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Products, Q.E.P. Ketron 1000 PEEK; Mitsubishi Chemical Holdings: Tokyo, Japan, 2014. [Google Scholar]
- Pisano, R.; Fissore, D.; Barresi, A.A. Heat Transfer in Freeze-Drying Apparatus. In Developments in Heat Transfer; Bernardes, D.M.A.D.S., Ed.; InTech: Casorezzo, Italy, 2011; p. 688. [Google Scholar]
- Ganguly, A.; Alexeenko, A.; Nail, S. Experimental Determination of the Key Heat Transfer Mechanisms in Pharmaceutical Freeze Drying. J. Pharm. Sci. 2013, 102, 1610–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.X.; Zhu, G.Q.; Yu, M.M.; Liang, Z.H. Experimental study on upward flame spread characteristics of external thermal insulation material under the influence of porosity. Case Stud. Therm. Eng. 2018, 12, 365–373. [Google Scholar] [CrossRef]










| Step No. | Time (hh:mm) | Temperature (°C) | Vacuum (mbar) | |
|---|---|---|---|---|
| Shelves | Condensor | |||
| 1 Loading | 00:01 | 20.0 | n/a | 1000.0 |
| 2 Freezing | 00:20 | 0.0 | n/a | 1000.0 |
| 3 Freezing | 02:10 | 0.0 | n/a | 1000.0 |
| 4 Freezing | 01:20 | −45.0 | n/a | 1000.0 |
| 5 Freezing | 3:00 | −45.0 | n/a | 1000.0 |
| 41 Evacuation | 01:00 | −45.0 | −85.0 | 0.066 |
| 42 Primary Drying | 70:00 | −25.0 | −85.0 | 0.066 |
| 43 Primary Drying | 02:00 | −25.0 | −85.0 | 0.066 |
| 92 Secondary Drying | 00:15 | 25.0 | −85.0 | 0.036 |
| 93 Secondary Drying | 08:00 | 25.0 | −85.0 | 0.036 |
| 94 Secondary Drying | 00:20 | 5.0 | −85.0 | 0.036 |
| 95 Storage | 00:01 | 5.0 | −85.0 | 0.036 |
| Position | Temperature (°C) |
|---|---|
| Top side of the rack | −15 |
| Bottom side of the rack | −23 |
| Shelf | −25 |
| Chamber wall | −8 |
| Product | −35 |
| Position | Temperature (°C) | End of Steady State Phase (h) | End of Primary Drying (h) | Difference between Corner and Center at the End of Primary Drying (%) | |
|---|---|---|---|---|---|
| During Steady State Phase | At the End of Primary Drying | ||||
| Rack | −20 | −15 | n/a | n/a | n/a |
| Shelf | −25 | −25 | n/a | n/a | n/a |
| Chamber wall | −8 | −8 | n/a | n/a | n/a |
| Separated vials corner | −33 | −15 | 20 | 27 | 40 |
| Separated vials center | −35 | −21 | 33 | 45 | |
| Rack corner | −33 | −18 | 25 | 33 | 27 |
| Rack center | −35 | −21 | 33 | 45 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daller, S.; Friess, W.; Schroeder, R. Energy Transfer in Vials Nested in a Rack System During Lyophilization. Pharmaceutics 2020, 12, 61. https://doi.org/10.3390/pharmaceutics12010061
Daller S, Friess W, Schroeder R. Energy Transfer in Vials Nested in a Rack System During Lyophilization. Pharmaceutics. 2020; 12(1):61. https://doi.org/10.3390/pharmaceutics12010061
Chicago/Turabian StyleDaller, Sarah, Wolfgang Friess, and Rudolf Schroeder. 2020. "Energy Transfer in Vials Nested in a Rack System During Lyophilization" Pharmaceutics 12, no. 1: 61. https://doi.org/10.3390/pharmaceutics12010061