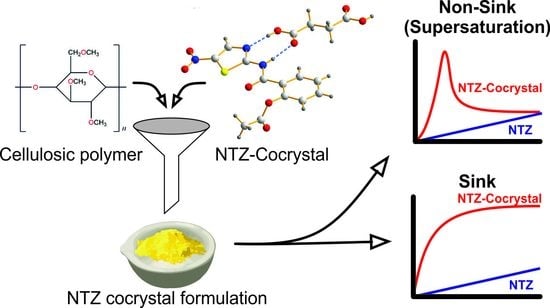
Dissolution Advantage of Nitazoxanide Cocrystals in the Presence of Cellulosic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NTZ Cocrystals
2.2.2. Solvent-Shift Method
2.2.3. Powder Dissolution under Non-Sink Conditions
2.2.4. Preparation of Powder Formulations with Methocel® 60 HG
2.2.5. Powder Dissolution Experiments in the USP 1 (Basket) Apparatus
2.3. Characterization Techniques
2.3.1. Powder X-Ray Diffraction Analysis (PXRD)
2.3.2. Scanning Electron Microscopy (SEM) Analysis
2.4. Statistical Analysis
3. Results
3.1. Preparation and Characterization of NTZ Cocrystals
3.2. Polymer Selection by Solvent-Shift Method
3.3. Powder Dissolution under Non-Sink Conditions
3.4. USP 1 Apparatus Powder Dissolution Experiments of Formulations with Polymer
4. Discussion
4.1. Performance of NTZ Cocrystals under Non-Sink Conditions
4.2. NTZ-SUC Cocrystal Formulation vs. Formulation with Pure NTZ
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rossignol, J.F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignol, J.F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health 2016, 9, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Shalan, S.; Nasr, J.J.; Belal, F. Determination of tizoxanide, the active metabolite of nitazoxanide, by micellar liquid chromatography using a monolithic column. Application to pharmacokinetic studies. Anal. Methods 2014, 6, 8682–8689. [Google Scholar] [CrossRef]
- Di Santo, N.; Ehrisman, J. A functional perspective of nitazoxanide as a potential anticancer drug. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 768, 16–21. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, H.J.; Song, C.S.; Choi, I.S.; Lee, J.B.; Park, S.Y. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int. Immunopharmacol. 2012, 13, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Jasenosky, L.D.; Cadena, C.; Mire, C.E.; Borisevich, V.; Haridas, V.; Ranjbar, S.; Nambu, A.; Bavari, S.; Soloveva, V.; Sadukhan, S. The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus. iScience 2019, 19, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, N.; Wood, R.D.; Welsh, W.J. Identification of Nitazoxanide as a Group I Metabotropic Glutamate Receptor Negative Modulator for the Treatment of Neuropathic Pain: An in Silico Drug Repositioning Study. Pharm. Res. 2015, 32, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Félix-Sonda, B.C.; Rivera-Islas, J.; Herrera-Ruiz, D.; Morales-Rojas, H.; Höpfl, H. Nitazoxanide Cocrystals in Combination with Succinic, Glutaric, and 2,5-Dihydroxybenzoic Acid. Cryst. Growth Des. 2014, 14, 1086–1102. [Google Scholar] [CrossRef]
- Matysiak-Budnik, T.; Mégraud, F.; Heyman, M. In-vitro transfer of nitazoxanide across the intestinal epithelial barrier. J. Pharm. Pharmacol. 2002, 54, 1413–1417. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Wouters, J.; Quéré, L. Pharmaceutical Salts and Co-Crystals; The Royal Society of Chemistry: Cambridge, UK, 2012; pp. 351–371. [Google Scholar]
- Kuminek, G.; Cao, F.; Rocha, A.B.O.; Cardoso, S.G.; Rodriguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Cavanagh, K.L.; Maheshwari, C.; Rodríguez-Hornedo, N. Understanding the Differences Between Cocrystal and Salt Aqueous Solubilities. J. Pharm. Sci. 2018, 107, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Rodriguez-Hornedo, N.; Amidon, G.E. Mechanistic Analysis of Cocrystal Dissolution, Surface pH, and Dissolution Advantage as a Guide for Rational Selection. J. Pharm. Sci. 2019, 108, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Kuminek, G.; Roy, L.; Cavanagh, K.L.; Yin, Q.; Rodríguez-Hornedo, N. Cocrystal Solubility Advantage Diagrams as a Means to Control Dissolution, Supersaturation, and Precipitation. Mol. Pharm. 2019, 16, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef] [PubMed]
- Almeida e Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Supersaturation Potential of Salt, Co-Crystal, and Amorphous Forms of a Model Weak Base. Cryst. Growth Des. 2016, 16, 737–748. [Google Scholar] [CrossRef]
- Xu, S.; Dai, W.G. Drug precipitation inhibitors in supersaturable formulations. Int. J. Pharm. 2013, 453, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Remenar, J.F.; Peterson, M.L.; Stephens, P.W.; Zhang, Z.; Zimenkov, Y.; Hickey, M.B. Celecoxib: Nicotinamide Dissociation: Using Excipients To Capture the Cocrystal’s Potential. Mol. Pharm. 2007, 4, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Kandi, P.; Lingireddy, S.R. Formulation of a Danazol Cocrystal with Controlled Supersaturation Plays an Essential Role in Improving Bioavailability. Mol. Pharm. 2013, 10, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; Ali, H.R.H.; Velaga, S.P. Effects of Polymer and Surfactant on the Dissolution and Transformation Profiles of Cocrystals in Aqueous Media. Cryst. Growth Des. 2014, 14, 643–648. [Google Scholar] [CrossRef]
- Li, M.; Qiu, S.; Lu, Y.; Wang, K.; Lai, X.; Rehan, M. Investigation of the Effect of Hydroxypropyl Methylcellulose on the Phase Transformation and Release Profiles of Carbamazepine-Nicotinamide Cocrystal. Pharm. Res. 2014, 31, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Ullah, H.; Murtaza, G.; Mahmood, Q.; Hussain, I. Evaluation of Influence of Various Polymers on Dissolution and Phase Behavior of Carbamazepine-Succinic Acid Cocrystal in Matrix Tablets. BioMed Res. Int. 2015, 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, M.; Hussain, I.; Sun, C.C. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev. Ind. Pharm. 2016, 42, 969–976. [Google Scholar] [CrossRef]
- Qiu, S.; Li, M. Effects of coformers on phase transformation and release profiles of carbamazepine cocrystals in hydroxypropyl methylcellulose based matrix tablets. Int. J. Pharm. 2015, 479, 118–128. [Google Scholar] [CrossRef]
- Qiu, S.; Lai, J.; Guo, M.; Wang, K.; Lai, X.; Desai, U.; Juma, N.; Li, M. Role of polymers in solution and tablet-based carbamazepine cocrystal formulations. CrystEngComm 2016, 18, 2664–2678. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Qiao, N.; Wang, K. Influence of Sodium Lauryl Sulfate and Tween 80 on Carbamazepine–Nicotinamide Cocrystal Solubility and Dissolution Behaviour. Pharmaceutics 2013, 5, 508–524. [Google Scholar] [CrossRef]
- Guo, M.; Wang, K.; Hamill, N.; Lorimer, K.; Li, M. Investigating the Influence of Polymers on Supersaturated Flufenamic Acid Cocrystal Solutions. Mol. Pharm. 2016, 13, 3292–3307. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Miyake, M.; Kawato, T.; Bando, M.; Toda, M.; Kato, Y.; Fukami, T.; Ozeki, T. Impact of the Dissolution Profile of the Cilostazol Cocrystal with Supersaturation on the Oral Bioavailability. Cryst. Growth Des. 2017, 17, 550–557. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Li, M.; Wang, J.R.; Mei, X. Modulating the Dissolution and Mechanical Properties of Resveratrol by Cocrystallization. Cryst. Growth Des. 2017, 17, 3989–3996. [Google Scholar] [CrossRef]
- Wang, C.; Tong, Q.; Hou, X.; Hu, S.; Fang, J.; Sun, C.C. Enhancing Bioavailability of Dihydromyricetin through Inhibiting Precipitation of Soluble Cocrystals by a Crystallization Inhibitor. Cryst. Growth Des. 2016, 16, 5030–5039. [Google Scholar] [CrossRef]
- Shimpi, M.R.; Alhayali, A.; Cavanagh, K.L.; Rodríguez-Hornedo, N.; Velaga, S.P. Tadalafil–Malonic Acid Cocrystal: Physicochemical Characterization, pH-Solubility, and Supersaturation Studies. Cryst. Growth Des. 2018, 18, 4378–4387. [Google Scholar] [CrossRef]
- Jasani, M.S.; Kale, D.P.; Singh, I.P.; Bansal, A.K. Influence of Drug–Polymer Interactions on Dissolution of Thermodynamically Highly Unstable Cocrystal. Mol. Pharm. 2019, 16, 151–164. [Google Scholar] [CrossRef]
- Kuminek, G.; Cavanagh, K.L.; da Piedade, M.F.M.; Rodríguez-Hornedo, N. Posaconazole Cocrystal with Superior Solubility and Dissolution Behavior. Cryst. Growth Des. 2019, 19, 6592–6602. [Google Scholar] [CrossRef]
- Sun, D.D.; Wen, H.; Taylor, L.S. Non-Sink Dissolution Conditions for Predicting Product Quality and in Vivo Performance of Supersaturating Drug Delivery Systems. J. Pharm. Sci. 2016, 105, 2477–2488. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, L.; Yao, J.; Ma, Y.Y.; Chen, J.M.; Lu, T.B. Improving the Solubility and Bioavailability of Apixaban via Apixaban–Oxalic Acid Cocrystal. Cryst. Growth Des. 2016, 16, 2923–2930. [Google Scholar] [CrossRef]
- Ferretti, V.; Dalpiaz, A.; Bertolasi, V.; Ferraro, L.; Beggiato, S.; Spizzo, F.; Spisni, E.; Pavan, B. Indomethacin Co-Crystals and Their Parent Mixtures: Does the Intestinal Barrier Recognize Them Differently? Mol. Pharm. 2015, 12, 1501–1511. [Google Scholar] [CrossRef]
- Liu, M.; Hong, C.; Yao, Y.; Shen, H.; Ji, G.; Li, G.; Xie, Y. Development of a pharmaceutical cocrystal with solution crystallization technology: Preparation, characterization, and evaluation of myricetin-proline cocrystals. Eur. J. Pharm. Biopharm. 2016, 107, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Weyna, D.R.; Cheney, M.L.; Shan, N.; Hanna, M.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Improving Solubility and Pharmacokinetics of Meloxicam via Multiple-Component Crystal Formation. Mol. Pharm. 2012, 9, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Mannava, M.C.; Nangia, A. Cocrystals and alloys of nitazoxanide: Enhanced pharmacokinetics. Chem. Commun. 2016, 52, 4223–4226. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Martinez-Pulgarin, D.F.; Muñoz-Urbano, M.; Gómez-Suta, D.; Sánchez-Duque, J.A.; Machado-Alba, J.E. Bibliometric Assessment of the Global Scientific Production of Nitazoxanide. Cureus 2017, 9, 1204. [Google Scholar] [CrossRef] [Green Version]
- Hotez, P.J. Could Nitazoxanide Be Added to Other Essential Medicines for Integrated Neglected Tropical Disease Control and Elimination? PLOS Negl. Trop. Dis. 2014, 8, 2758. [Google Scholar] [CrossRef] [Green Version]
- Aronson, H. Correction Factor for Dissolution Profile Calculations. J. Pharm. Sci. 1993, 82, 1190. [Google Scholar] [CrossRef]
- Guzmán, H.R.; Tawa, M.; Zhang, Z.; Ratanabanangkoon, P.; Shaw, P.; Gardner, C.R.; Chen, H.; Moreau, J.P.; Almarsson, Ö.; Remenar, J.F. Combined Use of Crystalline Salt Forms and Precipitation Inhibitors to Improve Oral Absorption of Celecoxib from Solid Oral Formulations. J. Pharm. Sci. 2007, 96, 2686–2702. [Google Scholar] [CrossRef]
- Chen, Y.M.; Rodríguez-Hornedo, N. Cocrystals Mitigate Negative Effects of High pH on Solubility and Dissolution of a Basic Drug. Cryst. Growth Des. 2018, 18, 1358–1366. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Kale, D.P.; Zode, S.S.; Bansal, A.K. Challenges in Translational Development of Pharmaceutical Cocrystals. J. Pharm. Sci. 2017, 106, 457–470. [Google Scholar] [CrossRef]
- Warren, D.B.; Benameur, H.; Porter, C.J.; Pouton, C.W. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. J. Drug Target. 2010, 18, 704–731. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, D.E.; Raina, S.; Zhou, D.; Gao, Y.; Zhang, G.G.; Taylor, L.S. Characterizing the Impact of Hydroxypropylmethyl Cellulose on the Growth and Nucleation Kinetics of Felodipine from Supersaturated Solutions. Cryst. Growth Des. 2012, 12, 1538–1547. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Liu, H.; Edgar, K.J.; Taylor, L.S. Impact of Polymers on Crystal Growth Rate of Structurally Diverse Compounds from Aqueous Solution. Mol. Pharm. 2013, 10, 2381–2393. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Takata, N.; Takano, R.; Hayashi, Y.; Terada, K. Dissolution Improvement and the Mechanism of the Improvement from Cocrystallization of Poorly Water-soluble Compounds. Pharm. Res. 2008, 25, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Jayasankar, A.; Reddy, L.S.; Bethune, S.J.; Rodríguez-Hornedo, N. Role of Cocrystal and Solution Chemistry on the Formation and Stability of Cocrystals with Different Stoichiometry. Cryst. Growth Des. 2009, 9, 889–897. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y.; Jain, P. HANDBOOK OF Aqueous Solubility Data; CRC Press: Boca Raton, FL, USA, 2010; pp. 96–154. [Google Scholar]











| Formulation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NTZ | 250 | 250 | 250 | 250 | ||||||||
| Phys. Mix. NTZ/SUC | 298 | 298 | 298 | 298 | ||||||||
| NTZ-SUC cocrystal | 298 | 298 | 298 | 298 | ||||||||
| Methocel® 60 HG b | 0 | 0 | 0 | 3.8 | 3.8 | 3.8 | 9.5 | 9.5 | 9.5 | 19 | 19 | 19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Zúñiga, R.; Rodríguez-Ruiz, C.; Höpfl, H.; Morales-Rojas, H.; Sánchez-Guadarrama, O.; Rodríguez-Cuamatzi, P.; Herrera-Ruiz, D. Dissolution Advantage of Nitazoxanide Cocrystals in the Presence of Cellulosic Polymers. Pharmaceutics 2020, 12, 23. https://doi.org/10.3390/pharmaceutics12010023
Salas-Zúñiga R, Rodríguez-Ruiz C, Höpfl H, Morales-Rojas H, Sánchez-Guadarrama O, Rodríguez-Cuamatzi P, Herrera-Ruiz D. Dissolution Advantage of Nitazoxanide Cocrystals in the Presence of Cellulosic Polymers. Pharmaceutics. 2020; 12(1):23. https://doi.org/10.3390/pharmaceutics12010023
Chicago/Turabian StyleSalas-Zúñiga, Reynaldo, Christian Rodríguez-Ruiz, Herbert Höpfl, Hugo Morales-Rojas, Obdulia Sánchez-Guadarrama, Patricia Rodríguez-Cuamatzi, and Dea Herrera-Ruiz. 2020. "Dissolution Advantage of Nitazoxanide Cocrystals in the Presence of Cellulosic Polymers" Pharmaceutics 12, no. 1: 23. https://doi.org/10.3390/pharmaceutics12010023