Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes
Abstract
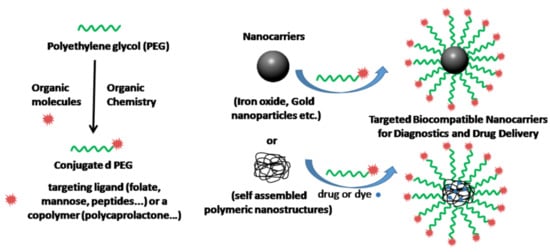
:1. Introduction
2. Synthesis of Modified Bio-Conjugated PEG Nanoparticles Acting as Targeting Agents
2.1. Poly(ε-caprolactone)-block-Poly(ethylene glycol)-Biotin (PCL-PEG-Biotin) Synthesis and Biomedical Application Overview
2.2. Magnetite-PEG-Folate Synthesis and Biomedical Application Overview
2.3. Poly(lactic-co-glycolic acid)-Block-Poly(ethylene glycol)-Mannose (PLGA-PEG-Mannose) Synthesis and Biomedical Application Overview
2.4. Poly(ε-caprolactone)-Block-Poly(ethylene glycol)-Small Molecular Ligand of Prostate Specific Membrane Antigen (PCL-PEG-SMLP) Synthesis and Biomedical Application Overview
2.5. Gold Nanopartilces-PEG-TAT Peptide (AuNp-PEG-TAT Peptide) Synthesis and Biomedical Application Overview
3. Synthesis of Modified Bio-Conjugated PEG Nanoparticles Acting as Tracking Agents
3.1. Coumarin-PEG-Au Synthesis and Biomedical Application Overview
3.2. TAMRA-PEG-Au Synthesis and Biomedical Application Overview
3.3. Near Infrared Conjugated PEG Nanoparticles
3.4. Graphene Oxide GO-PEG-Cy7 Synthesis and Biomedical Application Overview
3.5. Poly(L-leucine)-Block-Poly(ethylene glycol)-Block-Poly(L-leucine)(PLL-PEG-PLL) Synthesis and Biomedical Application Overview
4. Synthesis of Modified Bio-Conjugated PEG Nanoparticles Acting as Drug Carriers
4.1. Ibuprofen/PEG-Chitosan Synthesis and Biomedical Application Overview
4.2. Aspirin/Curcumin/mPEG-PLGA Synthesis and Biomedical Application Overview
4.3. Ridaforolimus/NH2-PEG-DSPE Synthesis and Biomedical Application Overview
4.4. Doxorubicin/MSN-Gelatin-PEG Synthesis and Biomedical Application Overview
4.5. Metal Organic Frameworks (MOFs) Synthesis and Biomedical Application Overview
4.6. DOX/MCN-PEG Synthesis and Biomedical Application Overview
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casettari, L.; Vllasaliu, D.; Mantovani, G.; Howdle, S.M.; Stolnik, S.; Illum, L. Effect of pegylation on the toxicity and permeability enhancement of chitosan. Biomacromolecules 2010, 11, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Kopeček, J. Soluble Synthetic Polymers as Potential Drug Carriers; Springer: Berlin/Heidelberg, Germany, 1984; pp. 51–101. [Google Scholar]
- Zhang, X.; Wang, H.; Ma, Z.; Wu, B. Effects of pharmaceutical pegylation on drug metabolism and its clinical concerns. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, C.; Güner, A. Solubility profiles of poly(ethylene glycol)/solvent systems, i: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Kobayashi, K.; Wei, J.; Iida, R.; Ijiro, K.; Niikura, K. Surface engineering of nanoparticles for therapeutic applications. Polym. J. 2014, 46, 460. [Google Scholar] [CrossRef]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; De Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of polyethyleneglycol (peg) chain length on the bio–nano-interactions between pegylated lipid nanoparticles and biological fluids: From nanostructure to uptake in cancer cells. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef]
- Shah, N.B.; Vercellotti, G.M.; White, J.G.; Fegan, A.; Wagner, C.R.; Bischof, J.C. Blood–nanoparticle interactions and in vivo biodistribution: Impact of surface peg and ligand properties. Mol. Pharm. 2012, 9, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Chan, W.C. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Papisov, M.I. Why do polyethylene glycol-coated liposomes circulate so long? Molecular mechanism of liposome steric protection with polyethylene glycol: Role of polymer chain flexibility au-torchilinl, v. P. J. Liposome Res. 1994, 4, 725–739. [Google Scholar]
- Saito, H.; Hoffman, A.S.; Ogawa, H.I. Delivery of doxorubicin from biodegradable peg hydrogels having schiff base linkages†. J. Bioact. Compat. Polym. 2007, 22, 589–601. [Google Scholar] [CrossRef]
- Working, P.K.; Newman, M.S.; Johnson, J.; Cornacoff, J.B. Safety of poly(ethylene glycol) and poly(ethylene glycol) derivatives. In Poly(Ethylene Glycol); American Chemical Society: Washington, DC, USA, 1997; Volume 680, pp. 45–57. [Google Scholar]
- Baumann, A.; Piel, I.; Hucke, F.; Sandmann, S.; Hetzel, T.; Schwarz, T. Pharmacokinetics, excretion, distribution, and metabolism of 60-kda polyethylene glycol used in bay 94-9027 in rats and its value for human prediction. Eur. J. Pharm. Sci. 2019, 130, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Faham, M.; Shokrollahi, H.; Yousefi, G.; Abbasi, S. Peg decorated glycine capped mn-ferrite nanoparticles synthesized by co-precipitation method for biomedical application. Adv. Mater. Res. 2014, 829, 274–278. [Google Scholar] [CrossRef]
- Bailey, F.E.; Koleske, J.V. Alkylene Oxides and Their Polymers; Dekker: New York, NY, USA, 1991; 261p. [Google Scholar]
- Kim, S.Y.; Cho, S.H.; Lee, Y.M.; Chu, L.-Y. Biotin-conjugated block copolymeric nanoparticles as tumor-targeted drug delivery systems. Macromol. Res. 2007, 15, 646–655. [Google Scholar] [CrossRef]
- Erdem, M.; Yalcin, S.; Gunduz, U. Folic acid-conjugated polyethylene glycol-coated magnetic nanoparticles for doxorubicin delivery in cancer chemotherapy: Preparation, characterization and cytotoxicity on hela cell line. Hum. Exp. Toxicol. 2017, 36, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.; Jain, N.K. Preparation, characterization and evaluation of targeting potential of amphotericin b-loaded engineered plga nanoparticles. Pharm. Res. 2009, 26, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sui, B.; Gou, J.; Liu, J.; Tang, X.; Xu, H.; Zhang, Y.; Jin, X. Psma ligand conjugated pcl-peg polymeric micelles targeted to prostate cancer cells. PLoS ONE 2014, 9, e112200. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, D.; Fu, W.; Li, J.; Crasto, C.; Jones, G.; DiMarzio, C.; Sridhar, S.; Amiji, M. Surface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and delivery. Int. J. Nanomed. 2006, 1, 51–57. [Google Scholar] [CrossRef]
- Dulkeith, E.; Ringler, M.; Klar, T.A.; Feldmann, J.; Muñoz Javier, A.; Parak, W.J. Gold nanoparticles quench fluorescence by phase induced radiative rate suppression. Nano Lett. 2005, 5, 585–589. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Hassani Najafabadi, A.; Abdouss, M.; Faghihi, S. Synthesis and evaluation of peg-o-chitosan nanoparticles for delivery of poor water soluble drugs: Ibuprofen. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 91–99. [Google Scholar] [CrossRef]
- Zhou, L.; Duan, X.; Zeng, S.; Men, K.; Zhang, X.; Yang, L.; Li, X. Codelivery of sh-aspirin and curcumin by mpeg-plga nanoparticles enhanced antitumor activity by inducing mitochondrial apoptosis. Int. J. Nanomed. 2015, 10, 5205–5218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R. Soc. Open Sci. 2018, 5, 170986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.K.; Ma, L.; Qiu, Y.; Xun, X.; Webster, T.J.; Su, M. Enhancing cancer radiation therapy with cell penetrating peptide modified gold nanoparticles. Austin J. Biomed. Eng. 2016, 3, 1033–1040. [Google Scholar]
- Hua, S.H.; Li, Y.Y.; Liu, Y.; Xiao, W.; Li, C.; Huang, F.W.; Zhang, X.Z.; Zhuo, R.X. Self-assembled micelles based on peg-polypeptide hybrid copolymers for drug delivery. Macromol. Rapid Commun. 2010, 31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Mandelbaum-Shavit, F.; Qazen, M.M.; Zalipsky, S. Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: In vitro studies. Bioconjug. Chem. 1999, 10, 289–298. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S. Ring-opening polymerization—An introductory review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Yu, G.C.; Yu, W.; Shao, L.; Zhang, Z.H.; Chi, X.D.; Mao, Z.W.; Gao, C.Y.; Huang, F.H. Fabrication of a targeted drug delivery system from a pillar[5]arene-based supramolecular diblock copolymeric amphiphile for effective cancer therapy. Adv. Funct. Mater. 2016, 26, 8999–9008. [Google Scholar] [CrossRef]
- Nosratia, H.; Barzegarib, P.; Danafar, H.; Manjilib, H.K. Biotin-functionalized copolymeric peg-pcl micelles for in vivo tumour-targeted delivery of artemisinin. Artif. Cells Nanomed. Biotechnol. 2019, 47, 104–114. [Google Scholar] [CrossRef]
- Maity, D.; Choo, S.-G.; Yi, J.; Ding, J.; Xue, J.M. Synthesis of magnetite nanoparticles via a solvent-free thermal decomposition route. J. Magn. Magn. Mater. 2009, 321, 1256–1259. [Google Scholar] [CrossRef]
- Yang, H.-M.; Lee, H.J.; Jang, K.-S.; Park, C.W.; Yang, H.W.; Heo, W.D.; Kim, J.-D. Poly(amino acid)-coated iron oxide nanoparticles as ultra-small magnetic resonance probes. J. Mater. Chem. 2009, 19, 4566–4574. [Google Scholar] [CrossRef]
- Yoo, M.K.; Park, I.K.; Lim, H.T.; Lee, S.J.; Jiang, H.L.; Kim, Y.K.; Choi, Y.J.; Cho, M.H.; Cho, C.S. Folate-peg-superparamagnetic iron oxide nanoparticles for lung cancer imaging. Acta Biomater. 2012, 8, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gendelman, H.E.; Zhang, G.; Puligujja, P.; McMillan, J.M.; Bronich, T.K.; Edagwa, B.; Liu, X.M.; Boska, M.D. Magnetic resonance imaging of folic acid-coated magnetite nanoparticles reflects tissue biodistribution of long-acting antiretroviral therapy. Int. J. Nanomed. 2015, 10, 3779–3790. [Google Scholar]
- Rajkumar, S.; Prabaharan, M. Multi-functional nanocarriers based on iron oxide nanoparticles conjugated with doxorubicin, poly(ethylene glycol) and folic acid as theranostics for cancer therapy. Colloid. Surf. B 2018, 170, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Bedane, K.G.; Singh, G.S. Reactivity and diverse synthetic applications of acyl isothiocyanates. Arkivoc 2015, 6, 206–245. [Google Scholar]
- Mitchell, J.P.; Roberts, K.D.; Langley, J.; Koentgen, F.; Lambert, J.N. A direct method for the formation of peptide and carbohydrate dendrimers. Bioorg. Med. Chem. Lett. 1999, 9, 2785–2788. [Google Scholar] [CrossRef]
- Kim, N.; Jiang, D.; Jacobi, A.M.; Lennox, K.A.; Rose, S.D.; Behlke, M.A.; Salem, A.K. Synthesis and characterization of mannosylated pegylated polyethylenimine as a carrier for sirna. Int. J. Pharm. 2012, 427, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Venier-Julienne, M.C.; Benoit, J.P. Preparation, purification and morphology of polymeric nanoparticles as drug carriers. Pharm. Acta Helv. 1996, 71, 121–128. [Google Scholar] [CrossRef]
- Zhu, S.J.; Niu, M.M.; O’Mary, H.; Cui, Z.R. Targeting of tumor-associated macrophages made possible by peg-sheddable, mannose-modified nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef]
- Gou, M.; Zheng, X.; Men, K.; Zhang, J.; Zheng, L.; Wang, X.; Luo, F.; Zhao, Y.; Zhao, X.; Wei, Y.; et al. Poly(epsilon-caprolactone)/poly(ethylene glycol)/poly(epsilon-caprolactone) nanoparticles: Preparation, characterization, and application in doxorubicin delivery. J. Phys. Chem. B 2009, 113, 12928–12933. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Hu, X.; Yan, L.; Chen, X.; Huang, Y.; Jing, X. Synthesis of biodegradable cationic triblock copolymer mpeg-pcl-pll for sirna delivery. J. Control. Release 2011, 152, e167–e168. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.P.; Elgamal, A.A.; Su, S.L.; Bostwick, D.G.; Holmes, E.H. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 1998, 83, 2259–2269. [Google Scholar] [CrossRef]
- Israeli, R.S.; Powell, C.T.; Corr, J.G.; Fair, W.R.; Heston, W.D. Expression of the prostate-specific membrane antigen. Cancer Res. 1994, 54, 1807–1811. [Google Scholar] [PubMed]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of peg-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharm. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Wang, F.H.; Wang, Y.; Zhang, X.; Zhang, W.J.; Guo, S.R.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef]
- Oh, E.; Delehanty, J.B.; Sapsford, K.E.; Susumu, K.; Goswami, R.; Blanco-Canosa, J.B.; Dawson, P.E.; Granek, J.; Shoff, M.; Zhang, Q.; et al. Cellular uptake and fate of pegylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano 2011, 5, 6434–6448. [Google Scholar] [CrossRef]
- De la Fuente, J.M.; Berry, C.C. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug. Chem. 2005, 16, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.; Conde, J.; Hernandez, Y.; Baptista, P.V.; Ibarra, M.R.; de la Fuente, J.M. Effect of peg biofunctional spacers and tat peptide on dsrna loading on gold nanoparticles. J. Nanopart. Res. 2012, 14, 917. [Google Scholar] [CrossRef]
- Su, W.W.; Wang, T.; Li, X.; Zhang, L.; Li, D.; Zuo, C. Iodine 131-labbled aunps-tat nanoparticles target cells nuclei in colon cancer for enhanced radioisotope therapy. J. Nucl. Med. 2019, 60, 1020. [Google Scholar]
- Li, X.; Zhang, X.-N.; Li, X.-D.; Chang, J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol. Med. 2016, 13, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokerst, J.V.; Gambhir, S.S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, B.; Ai, H. Theranostic nanoparticles for cancer and cardiovascular applications. Pharm. Res. 2014, 31, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tao, N. Detection, counting, and imaging of single nanoparticles. Anal. Chem. 2014, 86, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.-Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef]
- Bouzide, A.; Sauve, G. Silver(i) oxide mediated highly selective monotosylation of symmetrical diols. Application to the synthesis of polysubstituted cyclic ethers. Org. Lett. 2002, 4, 2329–2332. [Google Scholar] [CrossRef]
- Lukey, C.A. Thermoset coatings. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 9209–9215. [Google Scholar]
- Mai, C.; Elder, T. Wood: Chemically modified. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Heath, R. Chapter 28—Isocyanate-based polymers: Polyurethanes, polyureas, polyisocyanurates, and their copolymers. In Brydson’s Plastics Materials (Eighth Edition); Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 799–835. [Google Scholar]
- Lee, S.T.; Ramesh, N.S. Polymeric Foams: Mechanisms and Materials; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Mahou, R.; Wandrey, C. Versatile route to synthesize heterobifunctional poly(ethylene glycol) of variable functionality for subsequent pegylation. Polymers 2012, 4, 561. [Google Scholar] [CrossRef]
- Pelaz, B.; Grazu, V.; Ibarra, A.; Magen, C.; del Pino, P.; de la Fuente, J.M. Tailoring the synthesis and heating ability of gold nanoprisms for bioapplications. Langmuir 2012, 28, 8965–8970. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Chapter 1—Functional targets. In Bioconjugate Techniques (Second Edition); Hermanson, G.T., Ed.; Academic Press: New York, NY, USA, 2008; pp. 1–168. [Google Scholar]
- Hermanson, G.T. Chapter 2—The chemistry of reactive groups. In Bioconjugate Techniques (Second Edition); Hermanson, G.T., Ed.; Academic Press: New York, NY, USA, 2008; pp. 169–212. [Google Scholar]
- Tan, G.; Kantner, K.; Zhang, Q.; Soliman, M.G.; Del Pino, P.; Parak, W.J.; Onur, M.A.; Valdeperez, D.; Rejman, J.; Pelaz, B. Conjugation of polymer-coated gold nanoparticles with antibodies-synthesis and characterization. Nanomaterials (Basel) 2015, 5, 1297–1316. [Google Scholar] [CrossRef] [PubMed]
- Chance, B. Near-infrared images using continuous, phase-modulated, and pulsed light with quantitation of blood and blood oxygenation. Ann. N. Y. Acad. Sci. 1998, 838, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Hermanson, G.T. 2—The chemistry of reactive groups. In Bioconjugate Techniques; Hermanson, G.T., Ed.; Academic Press: San Diego, CA, USA, 1996; pp. 137–166. [Google Scholar]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Koenig, G.; Lohmar, E.; Rupprich, N.; Lison, M.; Gnass, A. Chloroacetic acids. Ullmann’s Encycl. Ind. Chem. 2012. [Google Scholar] [CrossRef]
- Daly, W.H.; Poché, D. The preparation of n-carboxyanhydrides of α-amino acids using bis(trichloromethyl)carbonate. Tetrahedron Lett. 1988, 29, 5859–5862. [Google Scholar] [CrossRef]
- Eckert, H.; Forster, B. Triphosgene, a crystalline phosgene substitute. Angew. Chem. Int. Ed. Engl. 1987, 26, 894–895. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, Y.; Zhi, X.; Du, H.; Yang, J. Controlled ring-opening polymerization of α-amino acid n-carboxy-anhydride by frustrated amine/borane Lewis pairs. Chem. Commun. 2017, 53, 5155–5158. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.; Hutchison, J. The mechanisms of polymerization of n-unsubstituted n-carboxyanhydrides1. J. Am. Chem. Soc. 1966, 88, 3627–3630. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. Pegylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Vicent, M.J.; Ringsdorf, H.; Duncan, R. Polymer therapeutics: Clinical applications and challenges for development. Adv. Drug Deliv. Rev. 2009, 61, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Deluca, P.P.; Lee, K.C. Emerging pegylated drugs. Expert Opin. Emerg. Drugs 2009, 14, 363–380. [Google Scholar] [CrossRef]
- Gaspar, R.; Duncan, R. Polymeric carriers: Preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv. Drug Deliv. Rev. 2009, 61, 1220–1231. [Google Scholar] [CrossRef]
- Hoste, K.; De Winne, K.; Schacht, E. Polymeric prodrugs. Int. J. Pharm. 2004, 277, 119–131. [Google Scholar] [CrossRef]
- Li, W.; Zhan, P.; De Clercq, E.; Lou, H.; Liu, X. Current drug research on pegylation with small molecular agents. Prog. Polym. Sci. 2013, 38, 421–444. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Torii, Y.; Ikeda, H.; Shimojoh, M.; Kurita, K.J.P.B. Chemoselective protection of chitosan by dichlorophthaloylation: Preparation of a key intermediate for chemical modifications. Polym. Bull. 2009, 62, 749. [Google Scholar] [CrossRef]
- Cai, G.; Jiang, H.; Tu, K.; Wang, L.; Zhu, K. A facile route for regioselective conjugation of organo-soluble polymers onto chitosan. Macromol. Biosci. 2009, 9, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Bruice, P.Y. Organic Chemistry, 8th ed.; Pearson: Upper Saddle River, NJ, USA, 2016; p. 443. [Google Scholar]
- Kulamarva, A.; Sebak, S.; Paul, A.; Bhathena, J.; Mirzaei, M.; Prakash, S. Ultrafine chitosan nanoparticles as an efficient nucleic acid delivery system targeting neuronal cells au-malhotra, meenakshi. Drug Dev. Ind. Pharm. 2009, 35, 719–726. [Google Scholar]
- Cheng, L.; Jin, C.; Lv, W.; Ding, Q.; Han, X. Developing a highly stable plga-mpeg nanoparticle loaded with cisplatin for chemotherapy of ovarian cancer. PLoS ONE 2011, 6, e25433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized plga-peg nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded plga nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Brandeis, E.; Newman, M.S.; Woodle, M.C. Long circulating, cationic liposomes containing amino-peg-phosphatidylethanolamine. FEBS Lett. 1994, 353, 71–74. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, R.; Zeng, Z.; Xu, L.; Wang, J. Application of poly(ethylene glycol)-distearoylphosphatidylethanolamine (peg-dspe) block copolymers and their derivatives as nanomaterials in drug delivery. Int. J. Nanomed. 2012, 7, 4185–4198. [Google Scholar]
- Remsberg, C.M.; Zhao, Y.; Takemoto, J.K.; Bertram, R.M.; Davies, N.M.; Forrest, M.L. Pharmacokinetic evaluation of a dspe-peg2000 micellar formulation of ridaforolimus in rat. Pharmaceutics 2012, 5, 81–93. [Google Scholar] [CrossRef]
- Yuan, R.; Kay, A.; Berg, W.J.; Lebwohl, D. Targeting tumorigenesis: Development and use of mtor inhibitors in cancer therapy. J. Hematol. Oncol. 2009, 2, 45. [Google Scholar] [CrossRef]
- Rivera, V.M.; Squillace, R.M.; Miller, D.; Berk, L.; Wardwell, S.D.; Ning, Y.; Pollock, R.; Narasimhan, N.I.; Iuliucci, J.D.; Wang, F.; et al. Ridaforolimus (ap23573; mk-8669), a potent mtor inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol. Cancer Ther. 2011, 10, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J. mTOR signaling and drug development in cancer. Nat. Rev. Clin. Oncol. 2010, 7, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S. A mesoporous silica nanosphere-based carrier system with chemically removable cds nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Zhao, J.; Kang, X.; Du, Y.; Yuan, X.; Hou, X. Preparation and properties of monolithic and hydrophobic gelatin–silica composite aerogels for oil absorption. J. Sol-Gel Sci. Technol. 2017, 83, 197–206. [Google Scholar] [CrossRef]
- Azami, M.; Rabiee, M.; Moztarzadeh, F. Glutaraldehyde crosslinked gelatin/hydroxyapatite nanocomposite scaffold, engineered via compound techniques. Polym. Compos. 2010, 31, 2112–2120. [Google Scholar] [CrossRef]
- Connolly, B.M.; Mehta, J.P.; Moghadam, P.Z.; Wheatley, A.E.H.; Fairen-Jimenez, D. From synthesis to applications: Metal-organic frameworks for an environmentally sustainable future. Curr. Opin. Green Sustain. Chem. 2018, 12, 47–56. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.L.; Li, D. Biological metal-organic frameworks: Structures, host-guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W.B. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Doonan, C.; Ricco, R.; Liang, K.; Bradshaw, D.; Falcaro, P. Metal-organic frameworks at the biointerface: Synthetic strategies and applications. Acc. Chem. Res. 2017, 50, 1423–1432. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.; et al. Controllable synthesis of dual-mofs nanostructures for ph-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.X.; Ni, K.Y.; Lin, W.B. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Li, Y.A.; Li, W.Y.; Dong, Y.B. Photodynamic therapy based on nanoscale metal-organic frameworks: From material design to cancer nanotherapeutics. Chem. Asian J. 2018, 13, 3122–3149. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.D.; Aung, T.; Guo, N.N.; Weichselbaum, R.; Lin, W.B. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, I.A.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.; Forgan, R.S. Selective surface pegylation of uio-66 nanoparticles for enhanced stability, cell uptake, and ph-responsive drug delivery. Chem 2017, 2, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, W.; Liu, R.; Zhang, J.; Zhang, D.; Li, Z.; Luan, Y. Rational design of metal organic framework nanocarrier-based codelivery system of doxorubicin hydrochloride/verapamil hydrochloride for overcoming multidrug resistance with efficient targeted cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 19687–19697. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Chen, X.R.; Zhang, L.; Ding, S.P.; Wang, X.; Lei, Q.F.; Fang, W.J. Fa-peg decorated mof nanoparticles as a targeted drug delivery system for controlled release of an autophagy inhibitor. Biomater. Sci. 2018, 6, 2582–2590. [Google Scholar] [CrossRef]
- Gimenez-Marques, M.; Bellido, E.; Berthelot, T.; Simon-Yarza, T.; Hidalgo, T.; Simon-Vazquez, R.; Gonzalez-Fernandez, A.; Avila, J.; Asensio, M.C.; Gref, R.; et al. Graftfast surface engineering to improve mof nanoparticles furtiveness. Small 2018, 14, 1801900. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, L.; Bian, X.; Kong, J.; Yang, P.; Liu, B. Ph-controlled delivery of doxorubicin to cancer cells, based on small mesoporous carbon nanospheres. Small 2012, 8, 2715–2720. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, F.; Qin, D.; Wang, Y.; Zhang, J.; Liu, J.; Feng, Y.; Lu, X. One-pot synthesis of silver nanoparticle catalysts supported on n-doped ordered mesoporous carbon and application in the detection of nitrobenzene. Carbon 2014, 69, 481–489. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujimoto, H.; Denayer, J.F.M.; Miyamoto, M.; Oumi, Y.; Miyake, Y. Surface modification of soft-templated ordered mesoporous carbon for electrochemical supercapacitors. Microporous Mesoporous Mater. 2015, 217, 141–149. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, D.; Zou, Y.; Wu, Z.; Li, F.; Che, R.; Deng, Y.; Tu, B.; Zhao, D. A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size. Angew. Chem. Int. Ed. Engl. 2010, 49, 7987–7991. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, T.; Wang, D.-W.; Lu, G.Q.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 2798. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Wu, M.; Meng, Q.; Chen, H.; Shu, Z.; Wang, J.; Zhang, L.; Li, Y.; Shi, J. Carbon nanocapsules: Colloidal rbc-shaped, hydrophilic, and hollow mesoporous carbon nanocapsules for highly efficient biomedical engineering. Adv. Mater. 2014, 26, 4293. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Liang, C.; Liu, Y.; Li, Z.; Yang, G.; Cheng, L.; Li, Y.; Liu, Z. Iron oxide @ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 2013, 7, 6782–6795. [Google Scholar] [CrossRef]
- Jung, S.H.; Jung, S.H.; Seong, H.; Cho, S.H.; Jeong, K.S.; Shin, B.C. Polyethylene glycol-complexed cationic liposome for enhanced cellular uptake and anticancer activity. Int. J. Pharm. 2009, 382, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Jie, P.; Venkatraman, S.S. Block copolymer ‘stealth’ nanoparticles for chemotherapy: Interactions with blood cells in vitro. Adv. Funct. Mater. 2008, 18, 716–725. [Google Scholar] [CrossRef]














| Nanoparticle System | Pathways for Their Synthesis | Biomedical Applications |
|---|---|---|
| PCL-PEG-Biotin [16] |
| Targeted paclitaxel chemotherapy drug to MCF-7 and HeLa cells. |
| Magnetite-PEG-Folate [17] |
| Targeted delivery of doxorubicin (DOX) to HeLa cells. |
| PLGA-PEG-Mannose [18] |
| Delivery of amphotericin B to macrophages via enhanced macrophage targeting and mannose-mannose uptake. |
| PCL-PEG-SMLP [19] |
| Specific delivery of four different (DTX-PMs) to a (PSMA) positive prostate LNCaP cells. |
| Coumarin-PEG-Gold [20] |
| Rapid internalization and intracellular tracking in MDA-MB-231 cells. |
| TAMRA-PEG-Gold [21] |
| Qualitative fluorescence imaging of the internalized AuNPs. |
| GO-PEG-CY7 [22] |
| In vivo fluorescence imaging in xenograft tumor mouse models. |
| Ibuprofen/Chitosan-PEG [23] |
| Encapsulation of ibuprofen, a poor water soluble drug, and in vitro release in gastrointestinal and simulated biological fluids. |
| Aspirin/Curcumin/PLGA-mPEG [24] |
| Synergistic anticancer effects on ES-2 and SKOV3 human ovarian carcinoma cells in vitro, and activation of the mitochondrial apoptosis pathway. |
| Doxorubicin/MSN-Gelatin-PEG [25] |
| In vitro improved cellular uptake and astonishing killing effectiveness to CD44-positive MDA-MB-231 cells. |
| AuNP-PEG-TAT Peptide [26] |
| Enhanced cellular uptake by Hela cells in vitro, and effectiveness in k generation of more oxygen reactive species resulting in cell death upon X-ray irradiation. |
| PLL-PEG-PLL [27] |
| Drug loading and in vitro drug release. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahme, K.; Dagher, N. Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics 2019, 11, 327. https://doi.org/10.3390/pharmaceutics11070327
Rahme K, Dagher N. Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics. 2019; 11(7):327. https://doi.org/10.3390/pharmaceutics11070327
Chicago/Turabian StyleRahme, Kamil, and Nazih Dagher. 2019. "Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes" Pharmaceutics 11, no. 7: 327. https://doi.org/10.3390/pharmaceutics11070327