1. Introduction
Life threatening diseases such as lung cancer, chronic obstructive pulmonary disease, and cardiovascular diseases (e.g., coronary heart disease and stroke) are usually associated with cigarette smoking (U.S. Department of Health and Human Services, 2014). However, these risks are significantly decreased when smoking is curtailed, depending on factors such as age, sex, physiology, and smoking frequency. For example, the life expectancy of a smoker after cessation at 35 years could increase by 20–24% in men and 17–22% in women. However, the life expectancy of a smoker after cessation at 65 years could increase by just 2–3% in men and 4–5% in women. It is therefore more beneficial to quit smoking as early as possible [
1]. An active smoker of tobacco draws in smoke known as mainstream smoke which comprises 8% tar and 92% gaseous components, while the side-stream released at the burning tail of a lit cigarette contains a higher proportion of poisonous components such as nitric oxide and carbon monoxide [
2], leading to risks of severe heart disease in non-smokers [
3].
Over the years, there have been various initiatives aimed at smoking cessation due to the health risks of tobacco, including the tobacco-free initiative (TFI) approved by the WHO. However, sudden cessation can lead to several withdrawal symptoms, including irritability, sleeplessness, and continuous cigarette craving [
4]. The withdrawal symptoms can last between 2 and 12 h [
5,
6] and have been managed using nicotine (NIC) replacement therapies (NRTs). NIC cannot be developed as an oral pill due to its susceptibility to first-pass metabolism in the liver [
7], and efforts have been made to develop alternative drug delivery systems [
8] for NRT. These include chewing gums, lozenges, mouth sprays, nasal sprays, transdermal patches (e.g., Nicorette), and oral films such as NiQuitin
® strips [
9]. Though these can achieve successful quitting of smoking, they have their limitations, and therefore novel delivery systems are required. The transdermal patch provides slow and sustained NIC release; however, it does not match the fast delivery of NIC from cigarette smoking [
10]. Itching, oedema, and erythema have also been associated with transdermal patches [
11]. NIC chewing gum can sometimes result in slow onset and prolonged plasma NIC levels which cannot be matched with the rapid pharmacological effect and high maximum arterial NIC levels required for relief [
12]. Oral sprays allow rapid NIC absorption, but they require constant administration, and hence the bioavailability at the therapeutic level is not sustained. NRT strips such as NiQuitin
® deals with the challenge of chewing gum for people with dental issues or who wear dentures. However, they still face the limitation of lower absorption of NIC due to the effect of eventual swallowing [
13]. Electronic NIC delivery systems (ENDS) can be misused as a substitute for cigarettes, encouraging significant numbers of smokers not to quit smoking altogether [
14]. Furthermore, a recent study has demonstrated the presence of low concentrations of free radicals in e-cigarettes, which can possibly cause harm to human cells [
15]. Lermer and co-workers also demonstrated the presence of potential cytotoxic metals (such as copper) and oxidants (e.g., perhydroxyl radical (HO
2·)) normally associated with conventional cigarette, in e-cigarettes [
16].
In comparison to transdermal routes, the buccal mucosa demonstrates better permeability since human buccal mucosa is composed mostly of non-keratinized cells [
17,
18]. The ready permeability of NIC across the buccal mucosa has been attributed to its high solubility in both water and organic solvents (LogP of 1.17) and its low molecular weight (162.2 g/mol) [
19,
20]. The permeability of NIC species depends on pH; however, all species of NIC can readily permeate mucosal membranes with higher permeation for un-ionized species than ionized species [
21]. Porcine buccal mucosa has similar morphology and permeation to the human buccal mucosa in terms of non-keratinized cells and enzymatic activities [
17], as reported for drugs such as naratriptan [
22], NIC [
23], buspirone [
24], omeprazole [
25,
26], and doxepin [
18]. Other buccal mucosa models such as sheep buccal mucosa have been reported in permeation studies [
27,
28]. EpiOral
TM buccal tissue comprises typical human-derived epithelial cells developed by MatTeK (MatTek Corporation, Ashland, MA, USA) and has recently been engineered and commercialized for better controlled permeation studies due to uniform and reproducible in vivo-like morphology and growth characteristics (
www.mattek.com (accessed 17 October 2016)). Several researchers have utilized EpiOral
TM buccal tissue as a model in permeation studies [
28,
29,
30].
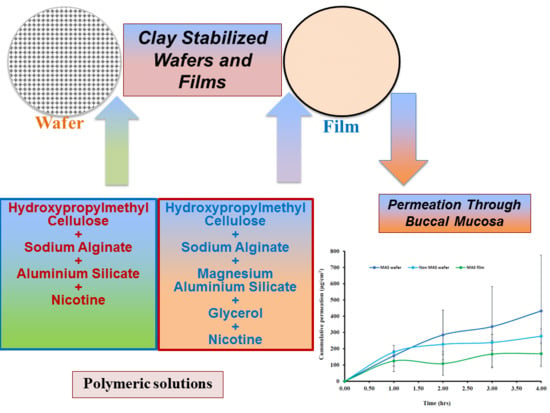
The aim of this study was to investigate the functional physicochemical characteristics of clay-stabilized NIC within composite wafers and films prepared from hydroxypropylmethylcellulose (HPMC) and sodium alginate (SA) compared with commercially available NIC-loaded strips. Further, the permeation of NIC released from the composite HPMC–SA wafers and films using porcine buccal tissue and a human equivalent EpiOralTM buccal tissue as well as the tissue viability of the EpiOralTM tissue after coming in contact with the NIC-loaded wafers and films was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
2. Materials and Methods
2.1. Materials
Hydroxypropylmethylcellulose (HPMC; Methocel K100 Premium LV) was a gift from Colorcon Limited (Dartford, UK), and magnesium aluminium silicate (MAS) was a gift from R.T. Vanderbilt Company Inc. (Norwalk, CT, USA). Sodium hydroxide, potassium dihydrogen phosphate, and gelatine were purchased from Fluka Analytical (Buchs, Switzerland). Nicotine (liquid form), sodium alginate (SA; molecular weight 120,000–190,000 g/mol, mannuronate/guluronate ratio 1.56), submaxillary mucin from porcine stomach, PBS tablets (pH 7.4), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Krebs–Ringer bicarbonate buffer, and dimethyl sulfoxide (DMSO) were all obtained from Sigma Aldrich (Dorset, UK). Sodium acetate, trimethylamine, and glycerol (GLY) were purchased from Fisher Scientific (Loughborough, UK). Commercially available NIC-loaded strips (NiQuitin®) was purchased from a local pharmacy (Gillingham, Kent). Calcium chloride dehydrate, sodium chloride, sodium phosphate dibasic, magnesium chloride hexahydrate, potassium carbonate hemihydrate, sodium phosphate monobasic monohydrate, sodium acetate, and trimethylamine were all purchased from Fisher Scientific (Loughborough, UK). The EpiOralTM buccal tissue kit (ORL-200) was purchased from MatTek Corporation (Ashland, MA, USA).
Preparation of Simulated Saliva
Simulated saliva was prepared using the formula summarized in
Table 1.
2.2. Selected Optimized Formulations
The following formulations in
Table 2 were selected for the investigation of the effect of PBS and SS (pH 6.8; ionic strength, 0.04) on swelling and in vitro drug dissolution characteristics. The MAS and non-MAS formulations were prepared as previously described, respectively [
32,
33].
2.3. Swelling and In Vitro Mucoadhesion Studies
The swelling capacities of wafers and films, as well as commercially available NIC-loaded strips, were determined by immersing each formulation into 5 mL of PBS (pH 6.8) or SS (pH 6.8; ionic strength, 0.04 M). The percentage swelling index was investigated by recording change in weight at time intervals of 2 min up to 30 min. For every time point, the medium was carefully removed to obtain an accurate weight of the sample and replaced with fresh medium. Three replicates were performed for each sample and swelling index (%) was calculated using Equation [
34].
where
Wd = dry weight of polymeric wafer/film,
Ws = weight of wafer/film after swelling.
Adhesion test was performed on the wafers and films using a TA. HD plus Texture Analyzer (Stable Micro Systems, Surry, UK) in tensile mode and fitted with a 5-kg load cell. Films were cut to dimensions matching the mathematical area of wafers (a circle with diameter = 15.5 mm) and attached to an adhesive probe (75 mm diameter) of the TA instrument using double-sided adhesive tape. Gelatine gel (6.67% (w/v)) was prepared by dissolving gelatine powder in water at 70 °C, poured into a Petri dish (86 mm diameter), and placed in a fridge overnight to set into a solid gel to represent the buccal mucosa surface. Mucin solution (2% w/v) was prepared by dissolving mucin powder in SS and 0.5 mL evenly spread on the surface of the set gelatine gel to simulate the buccal mucosa. The probe with film or wafer attached was lowered to make contact with the model buccal mucosa surface with an applied force of 1.0N and detached after a contact time of 60 s. Mucoadhesive strength was determined by the maximum adhesive force (Fmax) required to detach the sample from the model buccal surface, work of adhesion was determined by the area under the force-distance curve, while cohesiveness represents the distance the wafers/films travelled till they detached from the model buccal surface.
Finally, the swelling capacities and mucoadhesion profiles of the commercial NIC-loaded strip were compared to those of the formulated composite HPMC–SA wafers and films.
2.4. In Vitro Drug Dissolution Studies
In vitro drug dissolution of the wafers and films was performed with the help of a Franz-diffusion cell apparatus. The receptor compartment was filled with 8 mL of PBS or SS (pH 6.8) with a mesh on the receptor surface. The donor and receptor compartments were sealed with paraffin, to limit evaporation and held together by a pinch clamp. The system was placed on a water bath at 37 °C with magnetic stirring at approximately 200 rpm. The samples were weighed and placed on the mesh between the donor and receptor compartments. At predetermined time intervals, 0.5-mL aliquots of the dissolution media were withdrawn using a 1-mL syringe, filtered through a 0.45-µm cellulose acetate membrane, transferred into glass vials, and analysed using HPLC. The aliquots withdrawn at each time point, was replaced with fresh buffer solution, in order to maintain a constant volume of dissolution media. The percentage drug released from the formulations was calculated and plotted against time (n = 3). Further, the in vitro drug dissolution of the commercial strip was performed with the protocol described above but with only SS and compared to the drug release profiles of the HPMC–SA wafers and films.
2.5. Comparison of Release Profiles using Difference and Similarity Factors
The equations of Moore and Flanner were adopted in the calculation of the difference (
f1) and the similarity (
f2) factors in comparing the release profiles of the wafers and films in SS and PBS as well as between the wafers, films and commercial NIC-loaded strips. The difference factor value (
f1) measures the percent error between two curves over all time points, while the similarity (
f2) factors value is a logarithmic transformation of the sum-squared error of differences between the test
Tj and reference products
Rj over all time points [
35]. The difference (
f1) and the similarity (
f2) factors were calculated using Equations (2) and (3) below:
The drug release profiles are considered similar if the
f1 values is close to 0 and the
f2 values is close to 100 or if
f1 is lower than 15 and
f2 value is higher than 50. According to FDA recommendations, a similarity is declared for two drug release profiles if
f2 is between 50 and 100 [
36,
37,
38].
2.6. Drug Content (% Loading/Recovery)
The content of NIC in commercial strip was assayed and the results were compared to that obtained for the HPMC–SA wafers and films. The content of NIC in all the samples (n = 3) was assayed, by accurately weighing each sample and dissolving in 10 mL of distilled water. The resulting solution was collected into a syringe, filtered through a 0.45-µm cellulose acetate membrane, transferred into sample vials, placed in the HPLC sample chamber, and analysed using HPLC conditions as described below.
HPLC Analysis
NIC was analysed by HPLC using an Agilent 1200 HPLC instrument (Agilent Technologies, Cheshire, UK) with an auto sampler. The stationary phase used was a C-18 reverse-phase column, 4.6 × 250 mm (Phenomenex, Cheshire, UK). Sodium acetate solution, methanol and trimethylamine, (88:12:0.5
v/
v) were used as mobile phase with pH adjusted to 4.2 using glacial acetic acid, at a flow rate of 1 mL/min and UV detection at 259 nm [
39]. The retention time of NIC was detected at approximately 4.5 min. A calibration curve was plotted from NIC standards ranging from 40 µg/mL to 400 µg/mL (
R2 = 0.9994).
2.7. Permeation and Tissue Viability Studies
The HPMC–SA wafers and films were investigated for NIC permeation across porcine and EpiOralTM buccal tissues. Non-treated EpiOralTM tissue was used as a negative control in MTT assay for tissue integrity (viability) studies.
2.7.1. Ex Vivo Buccal Permeation Studies
Ex vivo buccal permeation studies of NIC released from wafers (MAS and non-MAS wafers) and films (MAS films) were carried out using Franz diffusion cell with a diffusional surface area of 0.6 cm
2. Fresh porcine buccal tissue was obtained from a local slaughterhouse (Kent, UK) and was immediately stored in a container containing Krebs–Ringer bicarbonate buffer (modified with sodium bicarbonate) and used within 2 h of slaughter [
28,
40]. The tissues were trimmed with a scalpel to a thickness of 1–3 mm and washed with physiological PBS (pH 6.8) at 37 °C. Membranes were mounted on a Franz diffusion cell between the donor and the receiver (8 mL of 0.01 M PBS; pH 6.8) compartments with the epithelial side facing the donor compartment. The receiver compartment was allowed to equilibrate at 37 °C for 30 min while stirring at 200–400 rpm. After the equilibration period, 0.5 mL of 0.01 M PBS were poured into the donor compartment and 20–30 mg of optimized wafers or films were placed in the donor compartment with the mucoadhesive layer in contact with the epithelial surface. The donor and the receiver chambers were held together tightly with a cell clamp and sealed with parafilm to limit evaporation. Samples (0.5 mL) were collected at predetermined time intervals from the port of the receiver compartment and replaced with the same amount of PBS in order to maintain a steady volume for 4 h. The collected samples were analysed using HPLC. Permeation flux (
J) was determined using Equation (4).
J = steady state flux; dQ/dt = amount of drug permeated; A = effective diffusion area.
2.7.2. In Vitro Buccal Permeation Studies (EpiOralTM Buccal Tissue)
EpiOralTM assay medium (MatTek, Ashland, MA, USA) was pre-warmed to 37 °C for 30 min. Using a sterile technique, 0.3 mL/well of EpiOralTM assay medium were pipetted into four wells of a 24-well plate and labelled as 1 h equilibration. The remaining wells were labelled as 0.5, 1, 2, 3, and 4 h. The EpiOralTM samples were transferred into the 1-h equilibration wells containing the pre-warmed assay medium and placed in a 37 °C, 5% CO2 incubator for 1 h. After 1 h equilibration, the EpiOralTM was transferred into the 0.5-h labelled well, treated with 0.5 mL donor solution (0.01 M PBS) into which 15 mg of wafers and/or film were added with the mucoadhesive layer in contact with the apical surface of the EpiOralTM buccal tissue, and returned to the incubator. After the elapsed time point (0.5 h) the tissue was moved to the next time point (i.e., 1, 2, 3, and 4 h) till the total elapsed time (4 h). Then, 50 μL of the receiver fluid were collected at predetermined time intervals and transferred to a vial for HPLC analysis. Permeation flux (J) was determined using Equation (4).
2.7.3. Permeation Correlation between Porcine and EpiOralTM Buccal Tissues.
The permeability of NIC across the porcine buccal tissue and EpiOralTM engineered human buccal tissue epithelium was further investigated to determine the correlation using a correlation curve of EpiOralTM cumulative permeation against the porcine cumulative permeation curve of wafers and film. Linear regression (R2) obtained from the curve of film and wafers was compared.
2.7.4. Tissue Viability (MTT Assay) of EpiOralTM Tissues after Permeation Studies
Following the EpiOralTM permeation studies, the tissue inserts used were transferred into a 24-well plate filled with MTT solution (0.3 mL) dissolved in PBS (5 mg/mL) and incubated for 3 h. After incubation, the MTT was gently extracted from all well plates and the cultures were extracted in 2 mL of DMSO for 2 h with gentle shaking (120 rpm). The aliquots (n = 3) of the extracts (200 μL) were placed in a 96-well plate and the absorbance of the extracted (purple-coloured) formazan was determined using a Multiskan microplate photometer at 540 nm. The viable cells had the greatest amount of MTT reduction and hence the highest absorbance values. Relative cell viability was calculated for each tissue used during permeation as a percentage of the mean negative control tissues (n = 3). The average percentage cell viability of optimized wafers and films was plotted using the non-treated tissues as negative control, which have 100% viability.
2.8. Statistical Analysis
Statistical analysis was performed using student t-test and/or one-way ANOVA to compare the results. The results were expressed as mean ± standard deviation and significant differences were determined at a level of p < 0.05.
4. Discussion
The design of a buccal drug delivery system involves the successful application of the optimized formulation to the buccal mucosa and the absorption of the drug either rapidly or in a controlled manner over a stipulated period. The immediate microenvironment of the buccal mucosa region plays a vital role in modulating the drug release with matrix swelling via hydration in the dissolution medium, diffusion and erosion of the polymer matrix as the main mechanisms of a controlled release formulation [
41,
42]. Human saliva therefore plays a major role in the release mechanism of a buccal drug delivery system and it is vital in functional characterization of swelling and in vitro release studies to consider the components i.e., presence of electrolytes such as sodium, calcium, potassium, chloride, phosphate, bicarbonate, and magnesium.
The swelling results demonstrated a significantly higher swelling index in PBS than SS (
p < 0.05) especially for MAS wafer. This implies that the presence of electrolytes and predominantly negatively charged mucin increases ionic interaction, which affected the swelling capacity of both optimized wafers and films. The diffusion of PBS into MAS wafer can be attributed to electro-osmosis i.e., generation of an electric field by mobile ions in MAS (silicate, magnesium, and aluminium) with accelerated flow, which induces high diffusivity of water molecules associated to these ions. This could also explain why MAS wafers demonstrated higher swelling capacity than non-MAS wafers. However, the presence of SS electrolytes reduced swelling capacity of the formulations by creating an ionic pressure gradient. This excess pressure was introduced with the difference in concentrations of ions in the formulation and in SS, which decreases the diffusion rate of SS into the formulations [
43]. Furthermore, the ions and mucin present in SS compete for available ionic interaction with SA and MAS in the MAS wafers and films and SA in optimized non-MAS wafer, hence reducing the rate of hydration [
26], and this eventually affects the rate of drug release from the swollen formulations.
The mucoadhesion of optimized wafers and films depends on various mechanisms of interaction with the mucosa surface such as adsorption, wetting, diffusion and mechanical theories [
44]. The mucoadhesion in the presence of SS is slightly lower than in PBS, as previously reported [
33] suggesting that the presence of higher ionic interactions by components in SS such as sodium, calcium, potassium, chloride, phosphate, bicarbonate, magnesium, and mucin could potentially interact with the negatively charged SA and MAS group. This limited the ionic interaction as well as hydrogen bonding with the mucin in the mucoadhesive model system used, because the ions and mucin present in SS compete for bonding sites on the wafer and film polymeric matrices [
45]. The high mucoadhesion in the films compared to wafers could be attributed to adhesion based on liquid-to-solid affinity (wetting theory), with the film’s large surface area playing a role in adhesion compared to optimized wafers with lower total surface area and lesser contact with the mucosal surface because of the presence of sponge-like pores. NiQuitin
® strips also followed similar mucoadhesion profiles as MAS films, since they are essentially a sheet-like formulation. However, the ionic effect on mucoadhesion from the high concentration of charged components in SS is minimal, with only methyl acrylic acid–ethyl acrylate copolymer (anionic) in NiQuitin
® strips as a competing site for ionic interaction of SS components compared to MAS film, which contained both SA (anionic polymer) and MAS (amphoteric clay). In addition, the high hydration and swelling properties of methyl acrylic acid–ethyl acrylate copolymer in NiQuitin
® improved the diffusion properties, which encouraged chain entanglement (diffusion theory of mucoadhesion).
One of the major challenges of dealing with free NIC base is its volatility, and NIC readily evaporates in an unstable formulation. Based on the conditions used in the analysis of the formulated wafers and film formulation, the low NIC content in NiQuitin® strips can be attributed to loss of NIC over time, including during analysis. This suggests that the hydrogen bonding between the copolymer and NIC was not stable enough to stabilize NIC within the commercial strips. The high NIC content in MAS formulation can be attributed to strong ionic interaction between the negatively charged silicate and the partially positively charged NIC in combination with hydrogen bonding between NIC and the composite polymers in the formulation, hence stabilizing NIC in the formulation.
The in vitro drug release from optimized wafers and films significantly depends on the hydration, which leads to swelling of the polymeric dosage form and eventual drug diffusion from the swollen matrix [
42]. As described above, the presence of electrolyte and mucin in SS caused a decrease in the swelling capacity of the formulated wafers and films. These components create an ionic pressure between the high concentration of charged components such as mucin and electrolyte in the SS and the ions in the formulation. At the molecular level, this initial slow rate of release could be related to ionic interactions between the drug, charged MAS, ionized alginate, and also competition with the ions present in SS as compared to PBS. This type of effect, especially in the presence of SS, has been reported in previous studies [
26], where release was significantly slower in SS than in PBS. It is possible that a longer period of release will show release of higher amounts of drug with eventual matrix erosion, and this will need to be investigated in future studies. However, for the purposes of buccal delivery, which was the main objective of the current study, the expected duration of release for effective permeation and systemic action is usually 2 h, since most of the drug will eventually end up in the saliva and be swallowed via the gastro-intestinal route.
The results of the in vitro drug release studies also demonstrated a similar trend to the swelling data with a decrease in the release profile of wafers and films formulation in SS as compared to the release profile in PBS. This implies that the presence of electrolytes and mucin slows down the release rate from the formulations over time, hence avoiding dumping of NIC in the buccal mucosa region [
26].
NiQuitin
® strip is composed mainly of anionic copolymers i.e., methacrylic acid–ethyl acrylate copolymer, and these copolymers contribute to the fast dissolution properties of NiQuitin
® formulation and the rapid release of NIC upon contact with saliva. Other components of NiQuitin
® include triethyl citrate used as a plasticizer, peppermint flavour and sucralose for taste masking, and sodium hydrogen carbonate used as a buffer, all of which have high affinity for water. The swelling profile (% swelling index against time) observed in NiQuitin
® strips showed that the maximum swelling profile of NiQuitin
® strips was attained within 6 min of contact with the SS medium. Methyl acrylic acid–ethyl acrylate is an anionic based copolymer that responds to environmental pH. Anionic hydrogels are usually ionized at higher pH above its pK
a and become un-ionized below its pK
a. The rapid dissolving process of the copolymer used in NiQuitin
® strips was activated with an increase in pH upon contact with the SS solution by the neutralizing base (sodium hydrogen carbonate) which then creates an osmotic swelling force in the copolymer network by the presence of hydroxyl ions [
46]. The rapid ingress of SS into the polymeric matric results in the eventual rapid erosion of the polymer matrix after its optimum swelling capacity as observed in NiQuitin
® swelling profile (
Figure 6). However, in the case of the MAS film and non-MAS wafer the swelling in SS was slower compared to the NiQuitin
®. Furthermore, the swelling appeared sustained in SS compared to PBS with the PBS profiles showing decrease in % swelling with time, suggesting that the erosion effects on MAS film and non-MAS wafer in SS was significantly lower than in NiQuitin
®. This could explain the significant differences observed in the eventual drug release in the first 2 h.
Compared with the HPMC–SA wafers and films, NiQuitin® strips showed a rapid release of NIC in less than 30 min. This can be attributed to the rapid swelling of the strips in response to environmental pH triggered by the neutralizing base in addition to the other highly water soluble components (triethyl citrate and sucralose). However, the sharp decrease in swelling of the NiQuitin® strips after the maximum swelling point at 6 min can be attributed to low concentration (8 mL of medium in receiving chamber) of sodium hydrogen carbonate which impacts on osmotic swelling force with a high ionic strength of the SS. The limitation of using the Franz diffusion cell to assess the release of NIC from formulations such as NiQuitin® is that the product is designed to be placed on the tongue and then pressed by the roof of the mouth. This increases the duration of drug release relative to actual application as the pressure applied on the strip by the roof of the mouth will have increased the disintegration of the polymer matrix and hence result in higher dissolution rate compared to the experimental results obtained in this study. The Franz diffusion cell used in this project could not model the pressure applied on the NiQuitin® both by the tongue and the roof of the mouth, but did demonstrate the dissolution of NiQuitin® upon contact with SS. Using FDA guidelines in comparing two or more dissolution profiles (similarity (f2) and difference (f1) factors), the wafers demonstrated a difference in dissolution with NiQuitin® as the MAS wafers and non-MAS wafers showed f2 similarity factor <15 and f1 difference factor <50, which could be related to the differences in micro- architecture.
This study also aimed to investigate NIC permeability when released from the wafers and films using porcine and human engineered EpiOral
TM buccal tissues as model buccal mucosa membranes. The toxicity of the buccal cells was assessed as the mucoadhesive formulations were loaded with NIC, which a known toxic compound at high doses [
47]. The buccal route offers an ideal opportunity for NIC delivery as it bypasses the NIC degradation (such as the hepatic first-pass effect) that occurs when administered by the conventional oral route. NIC has the ability to more easily penetrate the buccal route than the skin [
21,
48]. The buccal permeability of the wafers and films with different physicochemical properties and attributes such as swelling and release properties was necessary, as it is essential to achieve the required bioavailability for eventual therapeutic action. Furthermore, in order to assess the reliability of the permeation results, both porcine and human engineered EpiOral
TM buccal tissues were utilized and compared.
The most important properties that affect the permeability of a drug compound through a tissue membrane is its lipophilicity and molecular weight [
49]. Lipophilicity is usually expressed in terms of octanol–water partition coefficient (log
P). NIC possesses a low log
P value (1.17) and a low molecular weight of 162.23 g/mol, which make it highly permeable via the buccal mucosa at physiological pH (6.8), with un-ionized NIC permeating via the transcellular pathway, while ionized NIC permeates via the paracellular route [
18]. The permeation of NIC via porcine and EpiOral
TM buccal tissue in this study demonstrated a high flux value (between 40 μg/cm
2/h and 150 μg/cm
2/h) over a time period of 4 h. Similar high flux values of NIC have been observed in previous studies using porcine esophageal mucosa as a model membrane [
50] and human skin [
20]. Pongjanyakul and co-workers demonstrated in their permeation studies a high permeation curve of NIC between 400 μg/cm
2 and 800 μg/cm
2 within 8 h and also showed a decrease in film permeation rate as the MAS ratio in film increased, which was a similar case to this study as NIC permeation decreased in the MAS films [
50]. The use of porcine and EpiOral
TM buccal tissue in these studies demonstrated a good correlation. However, EpiOral
TM buccal tissue demonstrated a higher permeation flux (
J) than porcine buccal tissue, which can be attributed to fatty tissues beneath the ex vivo porcine buccal mucosa tissue.
Hydration, swelling, and release rate of NIC from the formulations played a role on the permeation flux across the porcine and EpiOral
TM buccal tissues. Wafer formulations (MAS and non-MAS wafers) showed a higher swelling index, which can be attributed to their high porosity, which allows rapid ingress of dissolution medium into the polymer matrix [
32]. The increased hydration and swelling of wafers played a role in the release rate of NIC from the polymer matrix which influenced the permeation flux in both porcine and EpiOral
TM buccal tissue models with higher flux in comparison to films. The increased NIC release from optimized wafers allows a higher concentration diffusion gradient towards absorption across the buccal membrane compared to the films. Film formulations (MAS films), on the other hand, demonstrated a lower permeation flux in both porcine and EpiOral
TM buccal tissue models which can be attributed to lower rates of hydration, swelling and release [
32,
51]. The diffusion of fluid into film formulation is relatively slow owing to the continuous polymeric sheet and absence of pores which therefore affects the hydration and swelling of the formulations and subsequent release rate of NIC from the swollen gels with low concentration diffusion gradient towards absorption across the buccal membrane. This implies that optimized wafers can provide a more rapid action while optimized films can provide a prolonged action.
MTT was utilized to assess the tissue viability of EpiOral
TM after contact with the optimized wafers and films. The assay investigates the reduction of yellow MTT to an insoluble purple formazan predominantly by enzymes (succinate dehydrogenase) found in the mitochondria of viable cells [
52,
53,
54]. The MTT assay demonstrated some level of cytotoxicity by NIC, with the non-viable cell’s inability to reduce yellow MTT to purple formazan. NIC has been reported by Chang and co-workers to suppress the growth of periodontal ligament fibroblast (PDLF) as well as inhibit cell proliferation and decrease protein synthesis, with an increase in NIC concentration [
47]. NIC-induced cytotoxicity was shown in the current study, with optimized wafers demonstrating 14% non-viable cells in MAS wafers and 19% non-viable cells in non-MAS wafers; however, a lower percentage of non-viable cells (8%) was shown in MAS films. The increase in NIC-induced cytotoxicity in optimized wafer formulations was as a result of increased hydration and swelling of wafers, which resulted in more rapid release of NIC from the polymeric matrix. The film formulation, however, demonstrated slow hydration and swelling, leading to slow NIC release with lower concentration diffusion gradient thereby resulting in lower numbers of non-viable cells in EpiOral
TM buccal tissue. However, in general, the results of cell viability (MTT) assay for both the wafers and films demonstrated that the formulations can be considered safe to use, since the percentage of viable cells was more than 70% after exposure, which is the acceptable international standard [
55,
56].