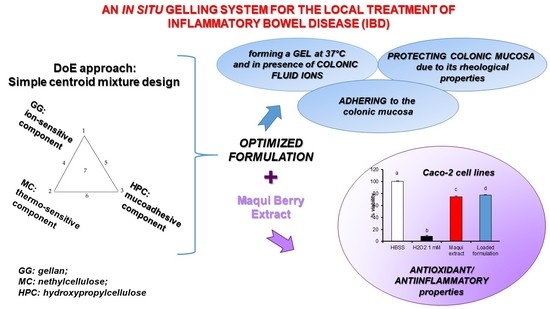
An In Situ Gelling System for the Local Treatment of Inflammatory Bowel Disease (IBD). The Loading of Maqui (Aristotelia chilensis) Berry Extract as an Antioxidant and Anti-Inflammatory Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Choice of the Gelling Agent
2.3. Influence of GG on MC Rheological Properties
2.4. DoE Approach: Simplex Centroid Mixture Design
2.4.1. Vehicle Characterization
Rheological Properties
Mucoadhesive Properties
2.5. Optimization Procedure
2.6. Maqui Berry Extract (MBE) Preparation
2.7. Preparation and Characterization of MBE-Loaded Optimized Vehicle (MBE-VH)
2.7.1. Anthocyanin Assay in MBE and MBE-Loaded Optimized Vehicle (MBE-VH)
2.7.2. In Vitro Evaluation of Biocompatibility and Antioxidant Properties of MBE-VH
MBE and MBE-VH Biocompatibility
MBE and MBE-VH Antioxidant Properties
3. Results and Discussion
3.1. Choice of the Gelling Agents
3.2. Experimental Design
3.3. Characterization of MBE-Loaded Optimized Vehicle (MBE-VH)
3.3.1. Anthocyanins Assay in MBE and MBE-Loaded Optimized Vehicle (MBE-VH)
3.3.2. In Vitro Evaluation of Biocompatibility and Antioxidant Properties of MBE-VH
Assessment of Fibroblast and Caco-2 Cells Viability Properties
Assessment of MBE Antioxidant Activity
Assessment of MBE-VH Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P. Importance of mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Regueiro, M. Extraintestinal Manifestations of Inflammatory Bowel Disease: Epidemiology, Etiopathogenesis, and Management. Curr. Gastroenterol. Rep. 2019, 21, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Irving, P.M. Optimization of conventional therapy in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Shahdadi Sardo, H.; Saremnejad, F.; Bagheri, S.; Akhgari, A.; Afrasiabi Garekani, H.; Sadeghi, F. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int. J. Pharm. 2019, 558, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Travis, S.P.L. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sinha, VR. Current pharmaceutical strategies for efficient site specific delivery in inflamed distal intestinal mucosa. J. Control. Release 2018, 272, 97–106. [Google Scholar] [CrossRef]
- Pásztor, E.; Makó, Á.; Csóka, G.; Fenyvesi, Z.; Benko, R.; Prosszer, M.; Marton, S.; Antal, I.; Klebovich, I. New formulation of in situ gelling Metolose-based liquid suppository. Drug Dev. Ind. Pharm. 2011, 37, 1–7. [Google Scholar] [CrossRef]
- Yuan, Y.; Ying, C.; Li, Z.; Hui-ping, Z.; Yi-Sha, G.; Bo, Z.; Xia, H.; Ling, Z.; Xiao-hui, W.; Li, C. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Deng, H.; Dong, A.; Song, J.; Chen, X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J. Control. Release 2019, 297, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Faccendini, A.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Gentile, M.; Ferrari, F. Development of a mucoadhesive and in situ gelling formulation based on κ-carrageenan for the treatment of the oral mucositis. I. A functional in vitro characterization. Mar. Drugs 2019, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Gentile, M.; Sandri, G.; Bonferoni, M.C.; Cavalloro, V.; Martino, E.; Collina, S.; Ferrari, F. Development of a Mucoadhesive and an in Situ Gelling Formulation Based on k-Carrageenan for Application on Oral Mucosa and Esophagus Walls. II. Loading of a Bioactive Hydroalcoholic Extract. Mar. Drugs 2019, 17, 153. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Faccendini, A.; Puccio, A.; Caramella, C. Comparison of poloxamer- and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis. Drug Dev. Ind. Pharm. 2014, 40, 352–360. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Singh, B.; Khurana, R.K.; Garg, B.; Saini, S.; Kaur, R. Stimuli-Responsive Systems with Diverse Drug Delivery and Biomedical Applications: Recent Updates and Mechanistic Pathways. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 209–255. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Lodge, T.P.; Maxwell, A.L.; Lott, J.R.; Schmidt, P.W.; McAllister, J.W.; Morozova, S.; Bates, F.S.; Li, Y.; Sammler, R.L. Gelation, Phase Separation, and Fibril Formation in Aqueous Hydroxypropylmethylcellulose Solutions. Biomacromolecules 2018, 19, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Faccendini, A.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Ferrari, F. Development of a mucoadhesive in situ gelling formulation for the delivery of Lactobacillus gasseri into vaginal cavity. Pharmaceutics 2019, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, K.; Saegusa, K.; Ishii, F. Rheological properties of reversible thermo-setting in situ gelling solutions with the methylcellulose–polyethylene glycol–citric acid ternary system (2): Effects of various water-soluble polymers and salts on the gelling temperature. Colloids Surf. B Biointerfaces 2009, 74, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Makó, Á.; Csóka, G.; Pásztor, E.; Marton, S.; Horvai, G.; Klebovich, I. Formulation of thermoresponsive and bioadhesive gel for treatment of oesophageal pain and inflammation. Eur. J. Pharm. Biopharm. 2009, 72, 260–265. [Google Scholar] [CrossRef]
- Forghani, A.; Devireddy, R. Methylcellulose Based Thermally Reversible Hydrogels. Methods Mol. Biol. 2018, 1773, 41–51. [Google Scholar]
- Demir Oğuz, Ö.; Ege, D. Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials 2018, 11, 604. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Parekh, H.B.; Rishad, J.; Jivani, N.P.; Patel, L.D.; Makwana, A.; Sameja, K. Novel in situ polymeric drug delivery system: A review. J. Drug Deliv. Ther. 2012, 2, 136–145. [Google Scholar]
- Adrover, A.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Trilli, J.; Cesa, S.; Tho, I.; Casadei, M.A. Gellan Gum/Laponite Beads for the Modified Release of Drugs: Experimental and Modeling Study of Gastrointestinal Release. Pharmaceutics 2019, 11, 187. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Akiyama, H.; Nakano, M.; Shoji, T.; Kanda, T.; Ohtake, Y.; Takita, T.; Matsuda, R.; Maitani, T. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int. Immunopharmacol. 2008, 8, 1802–1807. [Google Scholar] [CrossRef]
- Piberger, H.; Oehme, A.; Hofmann, C.; Dreiseitel, A.; Sand, P.G.; Obermeier, F.; Schoelmerich, J.; Schreier, P.; Krammer, G.; Rogler, G. Bilberries and their anthocyanins ameliorate experimental colitis. Mol. Nutr. Food Res. 2011, 55, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Mwinyia, J.; Scharla, M.; Freia, P.; Zeitzc, J.; Kullak-Ublickb, G.A.; Vavrickaa, S.R.; Frieda, M.; Webere, A.; Humpff, H.U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohn’s Colitis 2013, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, H.M.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Bosso, H.; Salzedas-Pescinini, L.M.; de Alvares Goulart, R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases? Green tea and inflammatory bowel diseases. J. Tradit. Complement. Med. 2018, 8, 267–277. [Google Scholar]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Suwalsky, M.; Vargas, P.; Avello, M.; Villena, F.; Sotomayor, C.P. Human erythrocytes are affected in vitro by flavonoids of Aristotelia chilensis (Maqui) leaves. Int. J. Pharm. 2008, 363, 85–90. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugnimolinae Turcz.): Souces of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Benatrehina, P.A.; Pan, L.; Naman, C.B.; Li, J.; Kinghorn, A.D. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement. Med. 2018, 8, 267–277. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.; Shirooie, S.; Sokeng, A.J.T.; et al. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (molina) stuntz) in a mouse model of Post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Schreckinger, M.; Wang, J.; Yousef, G.; Lila, M.; De Mejia, E. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vacciniumfloribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbæk, L.B.; Jebjerg, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Campieri, M.; Corbelli, C.; Gionchetti, P.; Brignola, C.; Belluzzi, A.; Di Febo, G.; Zagni, P.; Brunetti, G.; Miglioli, M.; Barbara, L. Spread and Distribution of 5-ASA Colonic Foam and 5-ASA Enema in Patients with Ulcerative Colitis. Dig. Dis. Sci. 1992, 37, 1890–1897. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Schiller, C.; Frohlich, C.-P.; Giessmann, T.; Siegmund, W.; Monnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Dejagher, B.; Vander Heyden, Y. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Mentori, I.; Boselli, C.; Cornaglia, A.I.; Daglia, M.; Marchese, A.; Caramella, C.; et al. Application of DoE approach in the development of mini-capsules, based on biopolymers and manuka honey polar fraction, as powder formulation for the treatment of skin ulcers. Int. J. Pharm. 2017, 516, 266–277. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef] [Green Version]
- Hibbert, D.B. Experimental design in chromatography: A tutorial review. J. Chromatogr. B 2012, 910, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, S.; Cirri, M.; Piepel, G.; Mennini, N.; Mura, P. Mixture experiment methods in the development and optimization of microemulsion formulations. J. Pharm. Biomed. Anal. 2011, 55, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Chlapanidas, T.; Torre, M.L.; Caramella, C. Sponge-like dressings based on the association of chitosan and sericin for the treatment of chronic skin ulcers. I. Design of experiments-assisted development. J. Pharm. Sci. 2016, 105, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Zerrouk, N.; Caramella, C. Mucoadhesive and penetration enhancement properties of three grades of hyaluronic acid using porcine buccal and vaginal tissue, Caco-2 cell lines, and rat jejunum. J. Pharm. Pharmacol. 2004, 56, 1083–1090. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis; Wiley & Sons: New York, NY, USA, 1981; pp. 412–419. [Google Scholar]
- Cornell, J.A. The original mixture problem: Design and models for exploring the entire simplex factor space. In Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data, 3rd ed.; Bloomfield, P., Cressie, N.A.C., Fisher, N.I., Johnstone, I.M., Kodane, J.R., Ryan, L.M., Scott, D.W., Silverman, B.W., Smith, A.F.M., Tengels, J.L., et al., Eds.; Wiley & Sons: New York, NY, USA, 2002; Volume 2, pp. 2–96. [Google Scholar]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Boselli, C.; Di Lorenzo, A.; Daglia, M.; Icaro Cornaglia, A.; Gioglio, L.; Perotti, C.; et al. Pectin/chitosan particles for the delivery of platelet lysate and manuka honey in chronic skin ulcers. Int. J. Pharm. 2016, 509, 59–70. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Daniel-da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517. [Google Scholar] [CrossRef]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hidrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 293–303. [Google Scholar] [CrossRef]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 2004, 108, 227–258. [Google Scholar] [CrossRef]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Tenci, M.; Del Fante, C.; Nicoletti, G.; Caramella, C. Sponge-Like Dressings Based on the Association of Chitosan and Sericin for the Treatment of Chronic Skin Ulcers. II. Loading of the Hemoderivative Platelet Lysate. J. Pharm. Sci. 2016, 105, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef] [PubMed]









| Points of Scheffè Triangle | GG % w/w | MC % w/w | HPC % w/w |
|---|---|---|---|
| 1 | 0.8 | 0 | 0 |
| 2 | 0 | 1 | 0 |
| 3 | 0 | 0 | 1 |
| 4 | 0.4 | 0.5 | 0 |
| 5 | 0.4 | 0 | 0.5 |
| 6 | 0 | 0.5 | 0.5 |
| 7 | 0.27 | 0.33 | 0.33 |
| Vehicles | Response Variables | ||||
|---|---|---|---|---|---|
| η 25 °C (Pa·s) | ΔIp | tgδ 37 °C | TA 37 °C (Pa·s−1) | ΔAUC/AUC 37 °C (mN·mm) | |
| 1 | 0.639 ± 0.005 a | 6.96 ± 0.13 a’ | 0.23 ± 0.16 a” | 290 ± 8 a* | 73 ± 22 a** |
| 2 | 0.258 ± 0.005 b | −0.47 ± 0.05 b’ | 0.28 ± 0.11 b” | −163 ± 29 b* | 18 ± 4 b** |
| 3 | 0.378 ± 0.012 c | −0.28 ± 0.06 c’ | 3.92 ± 0.35 c” | −14 ± 6 c* | 126 ± 18 c** |
| 4 | 0.1914 ± 0.0006 d | 7.15 ± 0.40 d ‘ | 0.09 ± 0.02 d” | −82 ± 12 d* | 32 ± 6 d** |
| 5 | 0.1842 ± 0.0006 e | 1.08 ± 0.14 e’ | 0.215 ± 0.005 e” | 65 ± 5e* | 79 ± 9 e** |
| 6 | 0.238 ± 0.001 f | −0.78± 0.03 f’ | 3.53 ± 0.14 f” | 23 ± 7 f* | 59 ± 19 f** |
| 7 | 0.179 ± 0.027 g | −0.940 ± 0.001 g’ | 1.42 ± 0.45 g” | 26 ± 3 g* | 95 ± 32 g** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenci, M.; Rossi, S.; Giannino, V.; Vigani, B.; Sandri, G.; Bonferoni, M.C.; Daglia, M.; Longo, L.M.; Macelloni, C.; Ferrari, F. An In Situ Gelling System for the Local Treatment of Inflammatory Bowel Disease (IBD). The Loading of Maqui (Aristotelia chilensis) Berry Extract as an Antioxidant and Anti-Inflammatory Agent. Pharmaceutics 2019, 11, 611. https://doi.org/10.3390/pharmaceutics11110611
Tenci M, Rossi S, Giannino V, Vigani B, Sandri G, Bonferoni MC, Daglia M, Longo LM, Macelloni C, Ferrari F. An In Situ Gelling System for the Local Treatment of Inflammatory Bowel Disease (IBD). The Loading of Maqui (Aristotelia chilensis) Berry Extract as an Antioxidant and Anti-Inflammatory Agent. Pharmaceutics. 2019; 11(11):611. https://doi.org/10.3390/pharmaceutics11110611
Chicago/Turabian StyleTenci, Marika, Silvia Rossi, Valentina Giannino, Barbara Vigani, Giuseppina Sandri, Maria Cristina Bonferoni, Maria Daglia, Luigi Maria Longo, Cristina Macelloni, and Franca Ferrari. 2019. "An In Situ Gelling System for the Local Treatment of Inflammatory Bowel Disease (IBD). The Loading of Maqui (Aristotelia chilensis) Berry Extract as an Antioxidant and Anti-Inflammatory Agent" Pharmaceutics 11, no. 11: 611. https://doi.org/10.3390/pharmaceutics11110611