Decellularized Lymph Node Scaffolding as a Carrier for Dendritic Cells to Induce Anti-Tumor Immunity
Abstract
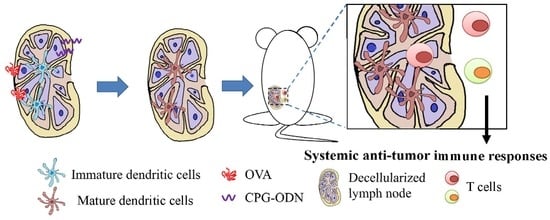
:1. Introduction
2. Materials and Methods
2.1. Decellularization of Lymph Nodes
2.2. The Microstructure of dLN Scaffolds
2.3. Biochemical Analysis and Histological Examination
2.4. Recellularization of BMDC into dLN Scaffolds
2.5. Stimulation of BMDC-dLN and the Cytokine Profile
2.6. Immunization of Mice and Tumor Challenge
2.7. Analysis of the Cell Populations inside the BMDC-dLN after Immunization
2.8. Ex Vivo Re-Stimulation of Splenocytes
2.9. Statistical Analysis
3. Results
3.1. The Efficacy of the Decellularization Process
3.2. Microstructure and ECM Examination of the dLN Scaffolds
3.3. Maturation Marker Analysis and Cytokines Released by BMDC-dLN
3.4. Protective Effect of the BMDC-dLN Vaccine against Tumor Challenge
3.5. Analysis of Cell Populations in BMDC-dLN after Immunization
3.6. Ex vivo Re-Stimulation of Splenocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Coulie, P.G.; Van den Eynde, B.J.; Agostinis, P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017, 38, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Feola, S.; Capasso, C.; Fusciello, M.; Martins, B.; Tahtinen, S.; Medeot, M.; Carpi, S.; Frascaro, F.; Ylosmaki, E.; Peltonen, K.; et al. Oncolytic vaccines increase the response to PD-L1 blockade in immunogenic and poorly immunogenic tumors. Oncoimmunology 2018, 7, e1457596. [Google Scholar] [CrossRef] [Green Version]
- Tacken, P.J.; de Vries, I.J.M.; Torensma, R.; Figdor, C.G. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007, 7, 790–802. [Google Scholar] [CrossRef]
- Schreibelt, G.; Bol, K.F.; Westdorp, H.; Wimmers, F.; Aarntzen, E.H.; Duiveman-de Boer, T.; van de Rakt, M.W.; Scharenborg, N.M.; de Boer, A.J.; Pots, J.M.; et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin. Cancer Res. 2016, 22, 2155–2166. [Google Scholar] [CrossRef]
- Kretz-Rommel, A.; Qin, F.; Dakappagari, N.; Torensma, R.; Faas, S.; Wu, D.; Bowdish, K.S. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J. Immunother. 2007, 30, 715–726. [Google Scholar] [CrossRef]
- Liu, Y.J.; Kanzler, H.; Soumelis, V.; Gilliet, M. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2001, 2, 585–589. [Google Scholar] [CrossRef]
- Ge, C.; Li, R.; Song, H.; Geng, T.; Yang, J.; Tan, Q.; Song, L.; Wang, Y.; Xue, Y.; Li, Z.; et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer 2017, 17, 884. [Google Scholar] [CrossRef]
- De Vries, I.J.; Krooshoop, D.J.; Scharenborg, N.M.; Lesterhuis, W.J.; Diepstra, J.H.; Van Muijen, G.N.; Strijk, S.P.; Ruers, T.J.; Boerman, O.C.; Oyen, W.J.; et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003, 63, 12–17. [Google Scholar] [PubMed]
- Eggermont, A.M. Therapeutic vaccines in solid tumours: Can they be harmful? Eur. J. Cancer 2009, 45, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, M.; Takakura, K.; Kanai, T.; Ito, Z.; Saito, K.; Takami, S.; Shimodaira, S.; Okamoto, M.; Ohkusa, T.; Koido, S. Dendritic cell-based cancer immunotherapy for colorectal cancer. World J. Gastroenterol. 2016, 22, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Itano, A.A.; Jenkins, M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003, 4, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Shimony, A.; Tidhar, D. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2008, 60, 228. [Google Scholar] [CrossRef] [PubMed]
- Cuzzone, D.A.; Albano, N.J.; Aschen, S.Z.; Ghanta, S.; Mehrara, B.J. Decellularized Lymph Nodes as Scaffolds for Tissue Engineered Lymph Nodes. Lymphat. Res. Biol. 2015, 13, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenti, E.; Bianchessi, S.; Proulx, S.T.; Palano, M.T.; Genovese, L.; Raccosta, L.; Spinelli, A.; Drago, D.; Andolfo, A.; Alfano, M.; et al. Therapeutic Regeneration of Lymphatic and Immune Cell Functions upon Lympho-organoid Transplantation. Stem Cell Rep. 2019, 12, 1260–1268. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Wang, T.; Li, T.; Chang, Y.; Sheu, M.T.; Huang, Y.Y.; Liu, D.Z. Development of Decellularized Cornea by Organic acid Treatment for Corneal Regeneration. Tissue Eng. Part A 2018. [Google Scholar] [CrossRef]
- Jiang, P.-L.; Lin, H.-J.; Wang, H.-W.; Tsai, W.-Y.; Lin, S.-F.; Chien, M.-Y.; Liang, P.-H.; Huang, Y.-Y.; Liu, D.-Z. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015, 11, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, M.; Labrecque, N. Murine superficial lymph node surgery. J. Vis. Exp. 2012, e3444. [Google Scholar] [CrossRef]
- Weber, J.S.; Mule, J.J. Cancer immunotherapy meets biomaterials. Nat. Biotechnol. 2015, 33, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Gabrielson, N.P.; Pack, D.W.; Jamison, R.D.; Wagoner Johnson, A.J. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials 2009, 30, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, M.; Kalbitzer, L.; Franz, S.; Moeller, S.; Schnabelrauch, M.; Simon, J.C.; Pompe, T.; Franke, K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv. Healthc. Mater 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.L.; et al. The effect of thick fibers and large pores of electrospun poly(epsilon-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef]
- Cheung, A.S.; Zhang, D.K.Y.; Koshy, S.T.; Mooney, D.J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 2018, 36, 160–169. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Bourland, J.; Rogic, A.; Skobe, M.; Auger, F.A. Tissue-engineered 3D human lymphatic microvascular network for in vitro studies of lymphangiogenesis. Nat. Protoc. 2017, 12, 1077–1088. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Warren Sands, R.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G.; et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef]
- Hoshiba, T.; Lu, H.; Kawazoe, N.; Chen, G. Decellularized matrices for tissue engineering. Exp. Opin. Biol. Ther. 2010, 10, 1717–1728. [Google Scholar] [CrossRef]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Song, J.J.; Guyette, J.P.; Gilpin, S.E.; Gonzalez, G.; Vacanti, J.P.; Ott, H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013, 19, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.A.; Hill, R.C.; Leiby, K.L.; Le, A.V.; Gard, A.L.; Madri, J.A.; Hansen, K.C.; Niklason, L.E. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 2016, 46, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Tan, F.J.; Marra, K.G.; Jan, S.S.; Liu, D.C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009, 5, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Lee, Y.K.; Shin, J.S.; Lim, K.J. Preparation of interconnected porous chitosan scaffolds by sodium acetate particulate leaching. J. Biomater. Sci. Polym. Ed. 2011, 22, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, R.N.; Jhan, H.J.; Liu, D.Z.; Ho, H.O.; Mao, Y.; Kohn, J.; Sheu, M.T. Development and Characterization of Acellular Extracellular Matrix Scaffolds from Porcine Menisci for Use in Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2015, 21, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Mathapati, S.; Bishi, D.K.; Guhathakurta, S.; Cherian, K.M.; Venugopal, J.R.; Ramakrishna, S.; Verma, R.S. Biomimetic acellular detoxified glutaraldehyde cross-linked bovine pericardium for tissue engineering. Mater. Sci. Eng. C 2013, 33, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.P.; Ritchie, S.C.; Hendrix, R.; Linsley, P.S.; Hathcock, K.S.; Hodes, R.J.; Lowry, R.P.; Pearson, T.C. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J. Immunol. 1994, 152, 5208–5219. [Google Scholar]
- Slaats, J.; Ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1beta/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Ann. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef]
- Fadel, T.R.; Sharp, F.A.; Vudattu, N.; Ragheb, R.; Garyu, J.; Kim, D.; Hong, E.; Li, N.; Haller, G.L.; Pfefferle, L.D.; et al. A carbon nanotube-polymer composite for T-cell therapy. Nat. Nanotechnol. 2014, 9, 639–647. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-J.; Wang, W.; Huang, Y.-Y.; Liao, W.-T.; Lin, T.-Y.; Lin, S.-Y.; Liu, D.-Z. Decellularized Lymph Node Scaffolding as a Carrier for Dendritic Cells to Induce Anti-Tumor Immunity. Pharmaceutics 2019, 11, 553. https://doi.org/10.3390/pharmaceutics11110553
Lin H-J, Wang W, Huang Y-Y, Liao W-T, Lin T-Y, Lin S-Y, Liu D-Z. Decellularized Lymph Node Scaffolding as a Carrier for Dendritic Cells to Induce Anti-Tumor Immunity. Pharmaceutics. 2019; 11(11):553. https://doi.org/10.3390/pharmaceutics11110553
Chicago/Turabian StyleLin, Hung-Jun, Weu Wang, Yi-You Huang, Wei-Tsen Liao, Ting-Yu Lin, Shyr-Yi Lin, and Der-Zen Liu. 2019. "Decellularized Lymph Node Scaffolding as a Carrier for Dendritic Cells to Induce Anti-Tumor Immunity" Pharmaceutics 11, no. 11: 553. https://doi.org/10.3390/pharmaceutics11110553