Anti-Inflammatory Effect of Cherry Extract Loaded in Polymeric Nanoparticles: Relevance of Particle Internalization in Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material and NP Preparation
2.2. Experimental Protocol
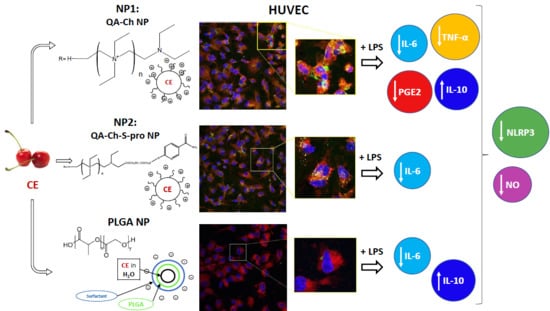
2.3. Measurement of Inflammatory Cytokines Production
2.4. NLRP3 Inflammasome Production
2.5. Nitric Oxide Assay
2.6. In Vitro Uptake Studies
2.7. Confocal Laser Scanning Microscopy (CLSM)
2.8. ImageStream®X Imaging Flow Cytometer
2.9. Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS)
2.10. Statistical Analysis
3. Results
3.1. Cherry Extract Characterization
3.2. FITC-Labeled NP Characterization
3.3. Cell Viability
3.4. Inflammatory Response
3.5. NLRP3 Inflammasome Production
3.6. In Vitro Uptake Studies
3.7. NP Quantification and Intracellular Localization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.; De Lima, R.; De Oliveira Assumpção, C.; Prestes, J.; Denadai, B.S. Consumption of cherries as a strategy to attenuate exercise-induced muscle damage and inflammation in humans. Nutr. Hosp. 2015, 32, 1885–1893. [Google Scholar] [CrossRef]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 7–17. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar] [CrossRef]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus Cerasus, L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Šarić, A.; Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Dragović-Uzelac, V.; Levaj, B.; Čosić, Z.; Šafranko, Ž.M.; Marotti, T. Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunus Cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mazza, G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-γ-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Cimino, F.; Molonia, M.S.; Ferrari, D.; Saija, A.; Virgili, F.; Speciale, A. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Nie, X.; Shi, J.; Liu, Q.; Wang, Z.; Li, X.; Zhou, J.; Su, J.; Xue, M.; Chen, W.D.; et al. Quercetin inhibits LPS-induced inflammation and ox-LDL-induced lipid deposition. Front. Pharmacol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Lietava, J.; Beerova, N.; Klymenko, S.V.; Panghyova, E.; Varga, I.; Pechanova, O. Effects of Cornelian Cherry on Atherosclerosis and Its Risk Factors. Oxid. Med. Cell Longev. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Felice, F.; Zambito, Y.; Belardinelli, E.; D’Onofrio, C.; Fabiano, A.; Balbarini, A.; Di Stefano, R. Delivery of natural polyphenols by polymeric nanoparticles improves the resistance of endothelial progenitor cells to oxidative stress. Eur. J. Pharm. Sci. 2013, 50, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Mattii, L.; Braca, A.; Felice, F.; Di Stefano, R.; Zambito, Y. Nanoparticles based on quaternary ammonium-chitosan conjugate: A vehicle for oral administration of antioxidants contained in red grapes. J. Drug Deliv. Sci. Technol. 2016, 32, 291–297. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Di Stefano, R.; Macedo, M.H.; Felice, F.; Zambito, Y.; Sarmento, B. Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles. Int. J. Mol. Sci. 2019, 20, 1759. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. To improve the resistance of endothelial cells to oxidative stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Uccello-Barretta, G.; Zaino, C.; Balzano, F.; Di Colo, G. Novel transmucosal absorption enhancers obtained by aminoalkylation of chitosan. Eur. J. Pharm. Sci. 2006, 29, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Fogli, S.; Zaino, C.; Stefanelli, F.; Breschi, M.C.; Di Colo, G. Synthesis, characterization and evaluation of thiolated quaternary ammonium-chitosan conjugates for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2009, 38, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. 2018, 130, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.-F.; Guerriero, G.; Cai, G. Functional Molecules in Locally-Adapted Crops: The Case Study of Tomatoes, Onions, and Sweet Cherry Fruits From Tuscany in Italy. Front. Plant Sci. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafezi, S. Antioxidant activities of iranian corn silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Tonutare, T.; Moor, U.; Szajdak, L. Strawberry anthocyanin determination by ph differential spectroscopic method – how to get true results? Acta. Sci. Pol. Hortorum. Cultus. 2014, 13, 35–47. [Google Scholar]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Bioanal. Chem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Sarmento, B.; Seabra, V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur. J. Pharm. Sci. 2018, 114, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Boggetto, N.; Contremoulins, V.; Mornet, S.; Reinhardt, N.; Marano, F.; Baeza-Squiban, A.; Boland, S. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part. Fibre Toxicol. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; Shahbazi, M.A.; Liu, D.; Fontana, F.; Correia, A.; Figueiredo, P.; Zhang, H.; Martins, J.P.; Hirvonen, J.T.; Granja, P.; et al. Nutlin-3a and Cytokine Co-loaded Spermine-Modified Acetalated Dextran Nanoparticles for Cancer Chemo-Immunotherapy. Adv. Funct. Mater. 2017, 27, 1–14. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Blanchard, C.L.; Oli, P.; Santhakumar, A.B. Pigmented Rice-Derived Phenolic Compounds Reduce Biomarkers of Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Mariathasan, S.; Monack, D.M. Inflammasome adaptors and sensors: Intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 2007, 7, 31–40. [Google Scholar] [CrossRef]
- Latz, E.; Duewell, P. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. 2018, 40, 61–73. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Y.; Wang, X.; Dong, Y.; Zheng, L.; Li, Y.; Ni, J. man in vivo and in vitro anti-inflammatory effects of ethanol fraction from Periploca forrestii Schltr. Chin. J. Integr. Med. 2017, 23, 528–534. [Google Scholar] [CrossRef]
- Tao, J.Y.; Zheng, G.H.; Zhao, L.; Wu, J.G.; Zhang, X.Y.; Zhang, S.L.; Huang, Z.J.; Xiong, F.L.; Li, C.M. Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells. J. Ethnopharmacol. 2009, 123, 97–105. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Laffleur, A.; Fischer, M.; Schmutzler, F.; Hintzen, A. Bernkop-Schnürch Evaluation of functional characteristics of preactivated thiolated chitosan as potential therapeutic agent for dry mouth syndrome. Acta. Biomater. 2015, 21, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Davda, J.; Davda, J.; Labhasetwar, V. Characterization of Nanoparticle Uptake by Endothelial Cells Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 2016, 233, 51–59. [Google Scholar] [CrossRef]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.X. Tight junction between endothelial cells: The interaction between nanoparticles and blood vessels. Beilstein J. Nanotechnol. 2016, 7, 675–684. [Google Scholar] [CrossRef]
- Perrone, M.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. S-preactivated thiolated glycol chitosan useful to combine mucoadhesion and drug delivery. Eur. J. Pharm. Biopharm. 2018, 132, 103–111. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2019, 126, 662–672. [Google Scholar] [CrossRef]








| Nanoparticle Type | Nanoparticle Size, nm | Polydispersity Index | ζ, mV |
|---|---|---|---|
| FITC-QA-Ch NPs (NP1) | 191.5 ± 3.7 | 0.30 ± 0.02 | 7.0 ± 0.9 |
| FITC-QA-Ch-S-pro NPs (NP2) | 235.4 ± 3.2 | 0.24 ± 0.02 | 5.5 ± 0.8 |
| FITC-PLGA NPs | 201.4 ± 5.7 | 0.06 ± 0.05 | −7.9 ± 0.1 |
| Population | Median | Std. Dev. |
|---|---|---|
| NP1 1 h | 4.961 | 1.488 |
| NP1 2 h | 4.486 | 1.148 |
| NP2 1 h | 4.307 | 1.545 |
| NP2 2 h | 3.754 | 1.269 |
| PLGA NPs 1 h | 3.235 | 0.8243 |
| PLGA NPs 2 h | 3.228 | 0.7129 |
| Population | Median | Std. Dev. |
|---|---|---|
| NP1 1 h | 118,514.27 | 60,578.46 |
| NP1 2 h | 159,675.65 | 67,120.87 |
| NP2 1 h | 86,177.00 | 51,031.86 |
| NP2 2 h | 124,872.98 | 78,847.77 |
| PLGA NPs 1 h | 72,220.92 | 45,585.81 |
| PLGA NPs 2 h | 85,886.73 | 52,101.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beconcini, D.; Felice, F.; Zambito, Y.; Fabiano, A.; Piras, A.M.; Macedo, M.H.; Sarmento, B.; Di Stefano, R. Anti-Inflammatory Effect of Cherry Extract Loaded in Polymeric Nanoparticles: Relevance of Particle Internalization in Endothelial Cells. Pharmaceutics 2019, 11, 500. https://doi.org/10.3390/pharmaceutics11100500
Beconcini D, Felice F, Zambito Y, Fabiano A, Piras AM, Macedo MH, Sarmento B, Di Stefano R. Anti-Inflammatory Effect of Cherry Extract Loaded in Polymeric Nanoparticles: Relevance of Particle Internalization in Endothelial Cells. Pharmaceutics. 2019; 11(10):500. https://doi.org/10.3390/pharmaceutics11100500
Chicago/Turabian StyleBeconcini, Denise, Francesca Felice, Ylenia Zambito, Angela Fabiano, Anna Maria Piras, Maria Helena Macedo, Bruno Sarmento, and Rossella Di Stefano. 2019. "Anti-Inflammatory Effect of Cherry Extract Loaded in Polymeric Nanoparticles: Relevance of Particle Internalization in Endothelial Cells" Pharmaceutics 11, no. 10: 500. https://doi.org/10.3390/pharmaceutics11100500