Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method
Abstract
:1. Introduction
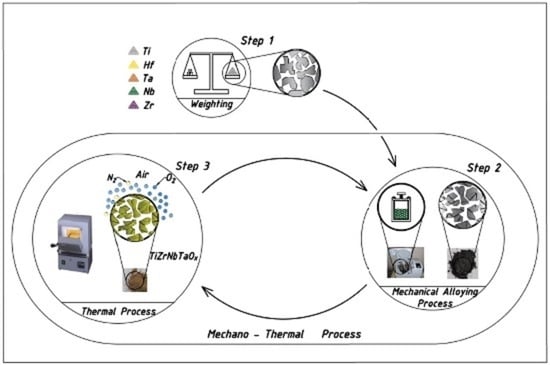
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Vo, D.V.B.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Hong, Q.; Cui, L.; Hong, P. The impact of carbon emissions trading on energy efficiency: Evidence from quasi-experiment in China’s carbon emissions trading pilot. Energy Econ. 2022, 110, 106025. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, A.F.; Cao, C.S.; Zhao, B. Applications of MOFs: Recent advances in photocatalytic hydrogen production from water. Coord. Chem. Rev. 2019, 390, 50–75. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Kim, Y.; Jun, S.E.; Lee, G.; Nam, S.; Jang, H.W.; Park, S.H.; Kwon, K.C. Recent Advances in Water-Splitting Electrocatalysts Based on Electrodeposition. Materials 2023, 16, 3044. [Google Scholar] [CrossRef]
- Piqué, O.; Illas, F.; Calle-Vallejo, F. Designing water splitting catalysts using rules of thumb: Advantages, dangers and alternatives. Phys. Chem. Chem. Phys. 2020, 22, 6797–6803. [Google Scholar] [CrossRef]
- Lee, G.; Jun, S.E.; Kim, Y.; Park, I.H.; Jang, H.W.; Park, S.H.; Kwon, K.C. Multicomponent Metal Oxide- and Metal Hydroxide-Based Electrocatalysts for Alkaline Water Splitting. Materials 2023, 16, 3280. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.N.; Shen, C.C.; Xu, A.W. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Moridon, S.T.N.; Arifin, K.; Mohamed, M.A.; Minggu, L.J.; Yunus, R.M.; Kassim, M.B. TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties. Materials 2023, 16, 6261. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Gawlak, K.; Popiolek, D.; Pisarek, M.; Sulka, G.D.; Zaraska, L. CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light. Materials 2022, 15, 3848. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Pooja Devi, P.; Basu, S. Revolutionizing the Role of Solar Light Responsive BiVO4/BiOBr Heterojunction Photocatalyst for the Photocatalytic Deterioration of Tetracycline and Photoelectrocatalytic Water Splitting. Materials 2023, 16, 5661. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fan, Z.; Nakabayashi, M.; Ju, H.; Pastukhova, N.; Xiao, Y.; Feng, C.; Shibata, N.; Domen, K.; Li, Y. Interface engineering of Ta3N5 thin film photoanode for highly efficient photoelectrochemical water splitting. Nat. Commun. 2022, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Edalati, P.; Shen, X.F.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. High-entropy oxynitride as a low-bandgap and stable photocatalyst for hydrogen production. J. Mater. Chem. A 2021, 9, 15076–15086. [Google Scholar] [CrossRef]
- Feng, X.; Feng, C.; Lu, Y. A Lightweight AlTiVNb High-Entropy Alloy Film with High Strength-Ductility Synergy and Corrosion Resistance. Materials 2022, 15, 8568. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y. History of high-entropy materials. In High-Entropy Materials: A Brief Introduction; Springer: Singapore, 2019; pp. 1–33. [Google Scholar]
- Edalati, P.; Itagoe, Y.; Ishihara, H.; Ishihara, T.; Emami, H.; Arita, M.; Fuji, M.; Edalati, K. Visible-light photocatalytic oxygen production on a high-entropy oxide by multiple-heterojunction introduction. J. Photochem. Photobiol. A Chem. 2022, 433, 114167. [Google Scholar] [CrossRef]
- Akrami, S.; Murakami, Y.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. Defective high-entropy oxide photocatalyst with high activity for CO2 conversion. Appl. Catal. B Environ. 2022, 303, 120896. [Google Scholar] [CrossRef]
- Edalati, P.; Wang, Q.; Razavi-Khosroshahi, H.; Fuji, M.; Ishihara, T.; Edalati, K. Photocatalytic hydrogen evolution on a high-entropy oxide. J. Mater. Chem. A 2020, 8, 3814–3821. [Google Scholar] [CrossRef]
- Seyyedin, S.; Zangi, H.; Bozorgmehr, M.; Ghasemi, B.; Tavallaei, M.M.; Adib, S. The effect of mechanical alloying time on the microstructural and mechanical properties of spark plasma sintered Ta–10W. Mater. Sci. Eng. A 2020, 798, 140024. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A critical review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Shundo, Y.; Watanabe, M.; Ishihara, T.; Fuji, M.; Edalati, K. Significant CO2 photoreduction on a high-entropy oxynitride. Chem. Eng. J. 2022, 449, 137800. [Google Scholar] [CrossRef]
- Rishel, D.; Smee, J.; Kammenzind, B. The corrosion behavior of hafnium in high-temperature water environments. J. Nucl. Mater. 2002, 303, 210–225. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Carrión, F.J.; Martínez-Nicolás, G.; López, R. Erosion–corrosion of stainless steels, titanium, tantalum and zirconium. Wear 2005, 258, 693–700. [Google Scholar] [CrossRef]
- Pan, T.; Chen, Y.; Zhang, B.; Hu, J.; Li, C. Corrosion behavior of niobium coated 304 stainless steel in acid solution. Appl. Surf. Sci. 2016, 369, 320–325. [Google Scholar] [CrossRef]
- Souza, J.C.; Apaza-Bedoya, K.; Benfatti, C.A.; Silva, F.S.; Henriques, B. A comprehensive review on the corrosion pathways of titanium dental implants and their biological adverse effects. Metals 2020, 10, 1272. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xu, J.; Zhou, J.; Wang, Y. Ultra-small yellow defective TiO2 nanoparticles for co-catalyst free photocatalytic hydrogen production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Qin, J.; Shen, X.; Yu, R.; Ma, M.; Liu, R. Porous carbon-doped TiO2 on TiC nanostructures for enhanced photocatalytic hydrogen production under visible light. J. Catal. 2017, 347, 36–44. [Google Scholar] [CrossRef]
- Esmat, M.; El-Hosainy, H.; Tahawy, R.; Jevasuwan, W.; Tsunoji, N.; Fukata, N.; Ide, Y. Nitrogen doping-mediated oxygen vacancies enhancing co-catalyst-free solar photocatalytic H2 production activity in anatase TiO2 nanosheet assembly. Appl. Catal. B Environ. 2021, 285, 119755. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, İ.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.C.; Ho, C.; Hou, Y.; Fu, X. Photocatalytic activity of a hierarchically macro/mesoporous titania. Langmuir 2005, 21, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Zhu, J.; Wang, S.; Cao, Y.; Qian, X.; Li, H. Self-assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase. J. Phys. Chem. C 2008, 112, 6258–6262. [Google Scholar] [CrossRef]
- Lo, A.Y.; Taghipour, F. Ordered mesoporous photocatalysts for CO2 photoreduction. J. Mater. Chem. A 2021, 9, 26430–26453. [Google Scholar] [CrossRef]











| Catalyst Name | Eg (eV) | BET Surface Area (m2/g) | Result (µmol/m2 h) |
|---|---|---|---|
| Ag2O/TiO2 | 2.86 | 24.65 | 135.90 |
| Au–TiO2 | 2.76 | 77.14 | 93.34 |
| GO–TiO2 | 2.58 | 42.72 | 88.95 |
| TiO2–ZnO | 3.06 | 85.91 | 15.02 |
| CdS | 2.40 | 24.57 | 48.84 |
| Au–CdS | 2.40 | 8.47 | 472.26 |
| CdS/Ti | 2.63 | 138.41 | 0.34 |
| Bi2S3/Pt/ZnO | 2.71 | 27.45 | 0.84 |
| ZnO/ZnS | 3.40 | 34.67 | 11.20 |
| In2O3/Ta2O5 | 2.80 | 43.75 | 12.18 |
| Rh/Cr2O3/GaZn | 2.60 | 48.30 | 30.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güler, Ö.; Boyrazlı, M.; Albayrak, M.G.; Güler, S.H.; Ishihara, T.; Edalati, K. Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method. Materials 2024, 17, 853. https://doi.org/10.3390/ma17040853
Güler Ö, Boyrazlı M, Albayrak MG, Güler SH, Ishihara T, Edalati K. Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method. Materials. 2024; 17(4):853. https://doi.org/10.3390/ma17040853
Chicago/Turabian StyleGüler, Ömer, Mustafa Boyrazlı, Muhammet Gökhan Albayrak, Seval Hale Güler, Tatsumi Ishihara, and Kaveh Edalati. 2024. "Photocatalytic Hydrogen Evolution of TiZrNbHfTaOx High-Entropy Oxide Synthesized by Mechano-Thermal Method" Materials 17, no. 4: 853. https://doi.org/10.3390/ma17040853