Navigating Pyrolysis Implementation—A Tutorial Review on Consideration Factors and Thermochemical Operating Methods for Biomass Conversion
Abstract
:1. Introduction
2. The Evolving Nature of Thermal Conversion Process
3. Potential Inter-Relatedness between Individual Components Affecting Pyrolysis-Based Research
4. Pre- to Post-Pyrolysis’ Engagement Strategies
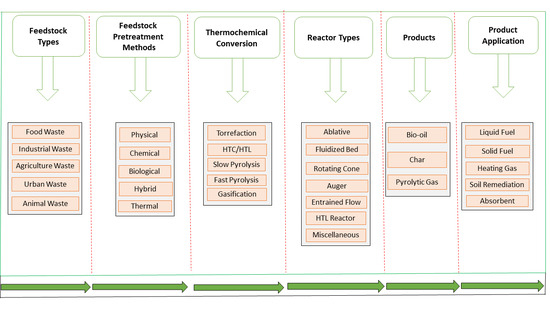
5. Potential Feedstock Employed in Thermal Conversion Processes
5.1. Feedstock Composition by Various Thermo-Chemical Reactors
5.2. Importance of Feedstock Composition in the Thermal Conversion Process
6. Major Pre-Treatment Strategies Applied to Feedstocks
- (a)
- Physical pretreatment
- (b)
- Chemical pretreatment
- (c)
- Thermal pretreatment
- (d)
- Biological pretreatment
- (e)
- Hybrid pretreatment
7. System Performance Considerations between Pyrolysis Reactors
7.1. Snapshots of The Single-Operated Pyrolysis Method
- (a)
- Fluidized bed reactor
- (b)
- Fixed bed
- (c)
- Thermogravimetric analysis (TGA)
- (d)
- Other peculiar pressure gas-based reactors
7.2. Snapshots of Combined Thermal Conversion Treatment and Analytical Methods
- (a)
- Fixed bed with torrefaction
- (b)
- Thermogravimetric analysis–Fourier transform infrared (TGA–FTIR)
- (c)
- Thermogravimetric analysis (TGA) and differential thermo-gravimetry (DTG)
- (d)
- TGA–FTIR and Py-GC/MS
- (e)
- Thermogravimetric analysis (TGA) and Pyrolysis-GC/MS
- (f)
- Other thermogravimetric analysis combinations
- (g)
- Analytical pyroprobe® reactor and Pyrolysis-GC/MS
7.3. Other Miscellaneous/Pyrolysis-Mimicking Operations
- (a)
- Hybrid organosolv–steam explosion reactor
- (b)
- Greenfield Eco. Pvt. Ltd./Cylindrical furnace reactor
- (c)
- Hydrothermal carbonization (HTC)
- (d)
- Other pyrolysis instruments
8. Differentiating between the Reactor and Operation Parameters Involved in Thermal Conversion Processes
8.1. Snapshots of Single-Operated Pyrolysis Method
8.2. Considerations of Residence Time and Particle Size
8.3. Considerations of Energy Demand
9. Knowledge Gaps and Future Prospects
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller analyzer |
| CSF | Carbonized solid fuel |
| CSTR | Continuous stirred tank reactor |
| DSC | Differential scanning calorimetry |
| DTG | Differential thermogravimetric analysis |
| FESEM | Field emission scanning electron microscopy |
| H/C | Hydrogen to carbon ratio |
| HHV | High heating value |
| HPLC-DAD | High performance liquid chromatography with photodiode-array detection |
| HTC/L | Hydrothermal carbonization/liquefaction |
| HTS | Hydrothermal treatment severity |
| LHV | Low heating value |
| MBMS | Molecular-beam mass spectrometry |
| MWSD | Mixed wood sawdust |
| O/C | Oxygen/carbon ratio |
| PCA | Principal component analysis |
| PFR | Plug flow reactor |
| Py-GCMS | Pyrolysis–gas chromatography–mass spectrometry |
| SPC | Safflower seed press cake |
| SVR | Support vector regression |
| TG/FTIR | Thermogravimetric analysis combined with Fourier transform infrared spectroscopy |
| TGA | Thermogravimetric analyzer |
| VFAs | Volatile fatty acids |
| WMR | Watermelon rind |
| XRD | X-ray diffraction |
References
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of Pyrolysis Processes in the Waste Management Sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Putra, P.H.M.; Rozali, S.; Patah, M.F.A.; Idris, A. A Review of Microwave Pyrolysis as a Sustainable Plastic Waste Management Technique. J. Environ. Manag. 2022, 303, 114240. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Andrew Lin, K.Y.; Hong, E.; Kwon, E.E.; Lee, J. The Valorization of Food Waste via Pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Golonka, M.; Scholz, M.; Białowiec, A. Opportunities and Challenges of High-pressure Fast Pyrolysis of Biomass: A Review. Energies 2021, 14, 5426. [Google Scholar] [CrossRef]
- Auersvald, M.; Macek, T.; Schulzke, T.; Staš, M.; Šimáček, P. Influence of Biomass Type on the Composition of Bio-Oils from Ablative Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2020, 150, 104838. [Google Scholar] [CrossRef]
- Basile, L.; Tugnoli, A.; Cozzani, V. The Role of Pressure in the Heat of Pyrolysis of a Lignocellulosic Biomass. Chem. Eng. Trans. 2015, 43, 451–456. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; do Lins, P.V.S.; Oliveira, L.M.T.d.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage Sludge-Derived Biochar for the Adsorptive Removal of Wastewater Pollutants: A Critical Review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Nor, W.; Wan, R.; Hisham, M.W.M.; Ambar, M.; Hin, T.Y. A Review on Bio-Oil Production from Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Huang, X.; Kudo, S.; Shusaku Asano, J.H. Improvement of Levoglucosenone Selectivity in Liquid Phase Conversion of Cellulose-Derived Anhydrosugar over Solid Acid Catalysts. Fuel Process. Technol. 2021, 212, 106625. [Google Scholar] [CrossRef]
- Naik, D.K.; Monika, K.; Prabhakar, S.; Parthasarathy, R.; Satyavathi, B. Pyrolysis of Sorghum Bagasse Biomass into Bio-Char and Bio-Oil Products: A Thorough Physicochemical Characterization Pyrolysis of Sorghum Bagasse Biomass into Bio-Char and Bio-Oil Products A Thorough Physicochemical Characterization. J. Therm. Anal. Calorim. 2017, 127, 1277–1289. [Google Scholar] [CrossRef]
- Michailof, C.M.; Kalogiannis, K.G.; Sfetsas, T.; Patiaka, D.T.; Lappas, A.A. Advanced Analytical Techniques for Bio-Oil Characterization. WIREs Energy Environ. 2016, 6, 614–639. [Google Scholar] [CrossRef]
- Pattiya, A. Bio-Oil Production via Fast Pyrolysis of Biomass Residues from Cassava Plants in a Fluidised-Bed Reactor. Bioresour. Technol. 2011, 102, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Sun, L.; Xiang, J.; Yang, T.; Zhang, A.; Zhang, J. Structural Evolution of Maize Stalk/Char Particles during Pyrolysis. Bioresour. Technol. 2009, 100, 4877–4883. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Eletta, O.A.A.; Adeniyi, A.G. Biomass Carbonisation in Retort Kilns: Process Techniques, Product Quality and Future Perspectives. Bioresour. Technol. Rep. 2022, 17, 100934. [Google Scholar] [CrossRef]
- Dupont, C.; Chiriac, R.; Gauthier, G.; Toche, F. Heat Capacity Measurements of Various Biomass Types and Pyrolysis Residues. Fuel 2014, 115, 644–651. [Google Scholar] [CrossRef]
- Elliott, D.C. Relation of Reaction Time and Temperature to Chemical Composition of Pyrolysis Oils; ACS Publications: Washington, DC, USA, 1988. [Google Scholar]
- Mahinpey, N.; Murugan, P.; Mani, T.; Raina, R. Analysis of Bio-Oil, Biogas, and Biochar from Pressurized Pyrolysis of Wheat Straw Using a Tubular Reactor. Energy Fuels 2009, 23, 2736–2742. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Influence of Pyrolysis Conditions on the Structure and Gasification Reactivity of Biomass Chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-ylijoki, J. Pyrolysis of Plastic Waste: Opportunities and Challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4959. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Liu, Z.; Chen, Y.; Yang, H.; Wang, X.; Che, Q.; Chen, W.; Chen, H. Pyrolysis Characteristics of Lignocellulosic Biomass Components in the Presence of CaO. Bioresour. Technol. 2019, 287, 121493. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Lin, C.H.; Wang, W.C. The Conversion of Biomass into Renewable Jet Fuel. Energy 2020, 201, 117655. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Pal, K.; Yehye, W.; Suresh, S.; Detection, H. Pyrolysis: A Sustainable Way to Generate Energy from Waste; IntechOpen: London, UK, 2017; Volume 69036, pp. 1–36. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y. Key Factors Influencing the Environmental Performance of Pyrolysis, Gasification and Incineration Waste-to-Energy Technologies. Energy Convers. Manag. 2019, 196, 497–512. [Google Scholar] [CrossRef]
- Lue, S. Principles and Advantages of Pyrolysis Moregreen. 2019, pp. 1–10. Available online: https://tomoregreen.com/principles-and-advantages-of-pyrolysis/ (accessed on 4 January 2024).
- Henan Doing Environmental Protection Technology Co., Ltd. What Is the Advantage and Disadvantage of Using Pyrolysis Technology to Convert Plastic to Oil? Henan Doing Environmental Protection Technology Co., Ltd.: Zhengzhou, China, 2019; pp. 1–4. [Google Scholar]
- Tian, B.; Xu, L.; Jing, M.; Liu, N.; Tian, Y. A Comprehensive Evaluation on Pyrolysis Behavior, Kinetics, and Primary Volatile Formation Pathways of Rice Husk for Application to Catalytic Valorization. Fuel Process. Technol. 2021, 214, 106715. [Google Scholar] [CrossRef]
- Kumar, P.; Subbarao, P.M.V.; Vijay, V.K. Assessment of Pyrolysis-Kinetics of Corncob and Eucalyptus Biomass Residue Using Thermo Gravimetric Analysis. Int. J. Sustain. Energy 2021, 40, 910–922. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Tsekos, C.; Retschitzegger, S.; Zimbardi, F.; Funke, A.; Banks, S.; Kraia, T.; Marques, P.; Scharler, R.; de Jong, W.; et al. Biomass Pyrolysis TGA Assessment with an International Round Robin. Fuel 2020, 276, 118002. [Google Scholar] [CrossRef]
- Su, Y.; Liu, L.; Zhang, S.; Xu, D.; Du, H.; Cheng, Y.; Wang, Z.; Xiong, Y. A Green Route for Pyrolysis Poly-Generation of Typical High Ash Biomass, Rice Husk: Effects on Simultaneous Production of Carbonic Oxide-Rich Syngas, Phenol-Abundant Bio-Oil, High-Adsorption Porous Carbon and Amorphous Silicon Dioxide. Bioresour. Technol. 2020, 295, 122243. [Google Scholar] [CrossRef]
- Matsakas, L.; Sarkar, O.; Jansson, S.; Rova, U.; Christakopoulos, P. A Novel Hybrid Organosolv-Steam Explosion Pretreatment and Fractionation Method Delivers Solids with Superior Thermophilic Digestibility to Methane. Bioresour. Technol. 2020, 316, 123973. [Google Scholar] [CrossRef]
- Téllez, J.F.; Silva, M.P.; Simister, R.; Gomez, L.D.; Fuertes, V.C.; De Paoli, J.M.; Moyano, E.L. Fast Pyrolysis of Rice Husk under Vacuum Conditions to Produce Levoglucosan. J. Anal. Appl. Pyrolysis 2021, 156, 105105. [Google Scholar] [CrossRef]
- Scapin, E.; Lazzari, E.; Benvenutti, E.V.; Falcade, T. Activated Carbon from Rice Husk Biochar with High Surface Area. Biointerface Res. Appl. Chem. 2021, 11, 10265–10277. [Google Scholar]
- Sieradzka, M.; Gao, N.; Quan, C.; Mlonka-Mędrala, A.; Magdziarz, A. Biomass Thermochemical Conversion via Pyrolysis with Integrated CO2 Capture. Energies 2020, 13, 1050. [Google Scholar] [CrossRef]
- Hao, J.; Qi, B.; Li, D.; Zeng, F. Catalytic Co-Pyrolysis of Rice Straw and Ulva Prolifera Macroalgae: Effects of Process Parameter on Bio-Oil up-Gradation. Renew. Energy 2021, 164, 460–471. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Li, S.; Wang, X.; Lin, R. Comparative Study on the Two-Step Pyrolysis of Different Lignocellulosic Biomass: Effects of Components. J. Anal. Appl. Pyrolysis 2020, 152, 104966. [Google Scholar] [CrossRef]
- Serio, M.A.; Wójtowicz, M.A. Methodology for Identification and Classification of Biomass Pyrolysis Behavior; SAE Technical Paper; Advanced Fuel Research, Inc.: East Hartford, CT, USA, 2009. [Google Scholar] [CrossRef]
- Noszczyk, T.; Dyjakon, A.; Koziel, J.A. Kinetic Parameters of Nut Shells Pyrolysis. Energies 2021, 14, 682. [Google Scholar] [CrossRef]
- Zhang, C.; Chao, L.; Zhang, Z.; Zhang, L.; Li, Q.; Fan, H.; Zhang, S.; Liu, Q.; Qiao, Y.; Tian, Y.; et al. Pyrolysis of Cellulose: Evolution of Functionalities and Structure of Bio-Char versus Temperature. Renew. Sustain. Energy Rev. 2021, 135, 110416. [Google Scholar] [CrossRef]
- Jia, G. Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis. Processes 2021, 9, 868. [Google Scholar] [CrossRef]
- Thamizhvel, R.; Suryavarman, K.; Velmurugan, V.; Sethuraman, N. Comparative Study of Gasification and Pyrolysis Derived from Coconut Shell on the Performance and Emission of CI Engine. Mater. Today Proc. 2021, 47, 978–983. [Google Scholar] [CrossRef]
- Swiechowski, K.; Koziel, J.A. The prediction of calorific value of carbonized solid fuel produced from refuse-derived fuel in the low-temperature pyrolysis in CO2. Materials 2020, 14, 49. [Google Scholar]
- Mu, L.; Wang, R.; Zhai, Z.; Zhang, B.; Shang, Y.; Yin, H. Evaluation of Thermokinetics Methodology, Parameters, and Coke Characterization of Co-Pyrolysis of Bituminous Coal with Herbaceous and Agricultural Biomass. Biomass Convers. Biorefinery 2021, 13, 5957–5972. [Google Scholar] [CrossRef]
- Matrapazi, V.K.; Zabaniotou, A. Experimental and Feasibility Study of Spent Coffee Grounds Upscaling via Pyrolysis towards Proposing an Eco-Social Innovation Circular Economy Solution. Sci. Total Environ. 2020, 718, 137316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, S.; Zhang, H.; Xiao, R. Fast Pyrolysis of Holocellulose for the Preparation of Long-Chain Ether Fuel Precursors: Effect of Holocellulose Types. Bioresour. Technol. 2021, 338, 125519. [Google Scholar] [CrossRef] [PubMed]
- Charvet, F.; Silva, F.; Ruivo, L.; Tarelho, L.; Matos, A.; da Silva, J.F.; Neves, D. Pyrolysis Characteristics of Undervalued Wood Varieties in the Portuguese Charcoal Sector. Energies 2021, 14, 2537. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of Agricultural Waste Biomass towards Production of Gas Fuel and High-Quality Char: Experimental and Numerical Investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Chen, Y.; Pattiya, A.; Yang, H.; Chen, H. Comparative Study of Wet and Dry Torrefaction of Corn Stalk and the e Ff Ect on Biomass Pyrolysis Polygeneration. Bioresour. Technol. 2018, 258, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Huang, H.; Evrendilek, F.; Wen, S.; Li, W. Optimizing Bioenergy and By-Product Outputs from Durian Shell Pyrolysis. Renew. Energy 2021, 164, 407–418. [Google Scholar] [CrossRef]
- Vinu, D.V.S.R. Effects of Biomass Particle Size on Slow Pyrolysis Kinetics and Fast Pyrolysis Product Distribution. Waste Biomass Valorization 2018, 9, 465–477. [Google Scholar] [CrossRef]
- Açıkalın, K. Evaluation of Orange and Potato Peels as an Energy Source: A Comprehensive Study on Their Pyrolysis Characteristics and Kinetics. Biomass Convers. Biorefinery 2021, 12, 501–514. [Google Scholar] [CrossRef]
- Moriyama, F.; Mizuno, S.; Tagami-Kanada, N.; Sawai, T. Evaluation of Energy Properties of Torrefied Biomass for a given Pyrolysis Condition by Isothermal Pyrolysis Kinetics. Mech. Eng. J. 2021, 8, 21-00069. [Google Scholar] [CrossRef]
- Ojha, D.K.; Viju, D.; Vinu, R. Fast Pyrolysis Kinetics of Lignocellulosic Biomass of Varying Compositions. Energy Convers. Manag. X 2021, 10, 100071. [Google Scholar] [CrossRef]
- Gözke, G.; Açıkalın, K. Pyrolysis Characteristics and Kinetics of Sour Cherry Stalk and Flesh via Thermogravimetric Analysis Using Isoconversional Methods. J. Therm. Anal. Calorim. 2020, 146, 893–910. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Tyagi, R.; Dalai, A.K.; Niu, C. Pyrolysis Kinetics and Activation Thermodynamic Parameters of Exhausted Coffee Residue and Coffee Husk Using Thermogravimetric Analysis. Can. J. Chem. Eng. 2021, 99, 1683–1695. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of Banana Leaves Biomass: Physico-Chemical Characterization, Thermal Decomposition Behavior, Kinetic and Thermodynamic Analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef] [PubMed]
- Shahbeig, H.; Nosrati, M. Pyrolysis of Biological Wastes for Bioenergy Production: Thermo-Kinetic Studies with Machine-Learning Method and Py-GC/MS Analysis. Fuel 2020, 269, 117238. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Zhao, R.; Song, G.; Tian, L. Pyrolysis of Phragmites Hirsuta Study on Pyrolysis Characteristics, Kinetic and Thermodynamic Analyses. Int. J. Energy Res. 2021, 45, 15200–15216. [Google Scholar] [CrossRef]
- da Silva, J.C.G.; de Albuquerque, J.G.; de Araujo Galdino, W.V.; de Sena, R.F.; Andersen, S.L.F. Single-Step and Multi-Step Thermokinetic Study—Deconvolution Method as a Simple Pathway for Describe Properly the Biomass Pyrolysis for Energy Conversion. Energy Convers. Manag. 2020, 209, 112653. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Chen, X.; Chen, Y.; Dong, Z.; Wang, X.; Yang, H. Comparative Pyrolysis Behaviors of Stalk, Wood and Shell Biomass: Correlation of Cellulose Crystallinity and Reaction Kinetics. Bioresour. Technol. 2020, 310, 123498. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of Biomass Type and Pyrolysis Temperature on Nitrogen in Biochar, and the Comparison with Hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Rodriguez Franco, C.; Page-Dumroese, D.S.; Pierson, D.; Miller, M.; Miles, T. Policy and Regulations for Mobile Biochar Production in the United States of America. Forests 2024, 15, 192. [Google Scholar] [CrossRef]
- Bahcivanji, L.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. The Effect of Post-Pyrolysis Treatment on Waste Biomass Derived Hydrochar. Waste Manag. 2020, 106, 55–61. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Wang, Z.; Wahia, H.; Mustapha, A.T.; Zhou, C.; Ma, H. Higher Heating Value, Exergy, Pyrolysis Kinetics and Thermodynamic Analysis of Ultrasound-Assisted Deep Eutectic Solvent Pretreated Watermelon Rind Biomass. Bioresour. Technol. 2021, 332, 125040. [Google Scholar] [CrossRef] [PubMed]
- Şen, U.; Pereira, H. Pyrolysis Behavior of Alternative Cork Species. J. Therm. Anal. Calorim. 2021, 147, 4017–4025. [Google Scholar] [CrossRef]
- Reinehr, T.O.; Ohara, M.A.; de Oliveira Santos, M.P.; Barros, J.L.M.; Bittencourt, P.R.S.; Baraldi, I.J.; da Silva, E.A.; Zanatta, E.R. Study of Pyrolysis Kinetic of Green Corn Husk. J. Therm. Anal. Calorim. 2021, 143, 3181–3192. [Google Scholar] [CrossRef]
- Pang, Y.X.; Yan, Y.; Foo, D.C.Y.; Sharmin, N.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. The Influence of Lignocellulose on Biomass Pyrolysis Product Distribution and Economics via Steady State Process Simulation. J. Anal. Appl. Pyrolysis 2020, 158, 104968. [Google Scholar] [CrossRef]
- Chen, D.; Gao, D.; Huang, S.; Capareda, S.C.; Liu, X.; Wang, Y.; Zhang, T.; Liu, Y.; Niu, W. Influence of Acid-Washed Pretreatment on the Pyrolysis of Corn Straw: A Study on Characteristics, Kinetics and Bio-Oil Composition. J. Anal. Appl. Pyrolysis 2021, 155, 105027. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam explosion of lignocellulosic biomass for multiple advanced bioenergy processes: A review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Mielke, K.; Kolb, T.; Müller, M. Chemical Fractionation of Inorganic Constituents in Entrained Flow Gasification of Slurry from Straw Pyrolysis. Biomass Bioenergy 2020, 141, 105732. [Google Scholar] [CrossRef]
- Chua, Y.W.; Wu, H.; Yu, Y. Effect of Cellulose-Lignin Interactions on Char Structural Changes during Fast Pyrolysis at 100-350 °C. Proc. Combust. Inst. 2021, 38, 3977–3986. [Google Scholar] [CrossRef]
- Fermoso, J.; Stevanov, C.; Moghtaderi, B.; Arias, B.; Pevida, C.; Plaza, M.G.; Rubiera, F.; Pis, J.J. High-Pressure Gasification Reactivity of Biomass Chars Produced at Different Temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 287–293. [Google Scholar] [CrossRef]
- Hussain, R.; Ghosh, K.K.; Ravi, K. Impact of Biochar Produced from Hardwood of Mesquite on the Hydraulic and Physical Properties of Compacted Soils for Potential Application in Engineered Structures. Geoderma 2021, 385, 114836. [Google Scholar] [CrossRef]
- Basile, L.; Tugnoli, A.; Stramigioli, C.; Cozzani, V. Influence of Pressure on the Heat of Biomass Pyrolysis. Fuel 2014, 137, 277–284. [Google Scholar] [CrossRef]
- Shrivastava, P.; Khongphakdi, P.; Palamanit, A.; Kumar, A.; Tekasakul, P. Investigation of Physicochemical Properties of Oil Palm Biomass for Evaluating Potential of Biofuels Production via Pyrolysis Processes. Biomass Convers. Biorefinery 2021, 11, 1987–2001. [Google Scholar] [CrossRef]
- Johansson, A.C.; Molinder, R.; Vikström, T.; Wiinikka, H. Particle Formation during Suspension Combustion of Different Biomass Powders and Their Fast Pyrolysis Bio-Oils and Biochars. Fuel Process. Technol. 2021, 218, 106868. [Google Scholar] [CrossRef]
- Rollag, S.A.; Lindstrom, J.K.; Brown, R.C. Pretreatments for the Continuous Production of Pyrolytic Sugar from Lignocellulosic Biomass. Chem. Eng. J. 2020, 385, 123889. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of Hydrochar Derived from Biomass—Experimental Investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar Pyrolysis of Waste Biomass: A Comparative Study of Products Distribution, in Situ Heating Behavior, and Application of Model-Free Kinetic Predictions. Fuel 2021, 292, 120365. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Sun, L. The Pyrolysis of Lignin: Pathway and Interaction Studies. Fuel 2021, 290, 120078. [Google Scholar] [CrossRef]
- Ringer, M.; Putsche, V.; Scahill, J. Large-Scale Pyrolysis Oil Production and Economic Analysis; Technical Report NREL/TP-510–37779; National Renewable Energy Laboratory: Cole Boulevard, CO, USA, 2006; pp. 1–93. [Google Scholar]
- Suntivarakorn, R.; Treedet, W.; Singbua, P.; Teeramaetawat, N. Fast Pyrolysis from Napier Grass for Pyrolysis Oil Production by Using Circulating Fluidized Bed Reactor: Improvement of Pyrolysis System and Production Cost. Energy Rep. 2018, 4, 565–575. [Google Scholar] [CrossRef]
- Wu, D.; Xiao, L.; Ba, Y.; Wang, H.; Zhang, A.; Wu, X.; Niu, M.; Fang, K. The Recovery of Energy, Nitrogen and Phosphorous from Three Agricultural Wastes by Pyrolysis. Energy Procedia 2017, 105, 1263–1269. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Zhang, Y.; Li, B.; Lu, H.; Duan, N.; Si, B.; Shen, R.; Lu, J. Recovery of Reducing Sugars and Volatile Fatty Acids from Cornstalk at Different Hydrothermal Treatment Severity. Bioresour. Technol. 2016, 199, 220–227. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Cotana, F.; Nicolini, A.; Coccia, V.; Petrozzi, A.; Formica, A.; Bertini, A. Characterization of Various Biomass Feedstock Suitable for Small-Scale Energy Plants as Preliminary Activity of Biocheaper Project. Sustainability 2020, 12, 6678. [Google Scholar] [CrossRef]
- Lu, X.; Ma, X.; Chen, X. Co-Hydrothermal Carbonization of Sewage Sludge and Lignocellulosic Biomass: Fuel Properties and Heavy Metal Transformation Behaviour of Hydrochars. Energy 2021, 221, 119896. [Google Scholar] [CrossRef]
- Saengsuriwong, R.; Onsree, T.; Phromphithak, S.; Tippayawong, N. Conversion of Tobacco Processing Waste to Biocrude Oil via Hydrothermal Liquefaction in a Multiple Batch Reactor. Clean Technol. Environ. Policy 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, I.; Naqvi, S.R.; Li, K.; Mehran, M.T.; Khoja, A.H.; Alarabi, A.A.; Atabani, A.E. Investigation of Slow Pyrolysis Mechanism and Kinetic Modeling of Scenedesmus Quadricauda Biomass. J. Anal. Appl. Pyrolysis 2021, 158, 105149. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Ahmed, A.; Saeed, S. Comparison of Synthetic and Low-Cost Natural Zeolite for Bio-Oil Focused Pyrolysis of Raw and Pretreated Biomass. J. Clean. Prod. 2021, 313, 127760. [Google Scholar] [CrossRef]
- Zheng, A.; Xia, S.; Cao, F.; Liu, S.; Yang, X.; Zhao, Z.; Tian, Y.; Li, H. Directional Valorization of Eucalyptus Waste into Value-Added Chemicals by a Novel Two-Staged Controllable Pyrolysis Process. Chem. Eng. J. 2021, 404, 127045. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.; Zhao, W.; Xu, L.; Bai, L. Pyrolysis Behaviors, Kinetics and Gaseous Product Evolutions of Two Typical Biomass Wastes. Catal. Today 2021, 374, 77–85. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, Y.; He, R.; Zheng, Z. Selective Conversion of Lignocellulosic Biomass and Its Components into Value-Added Furans over Al-Based Bimetals: Analytical Py-GC × GC/MS. J. Anal. Appl. Pyrolysis 2022, 163, 105485. [Google Scholar] [CrossRef]
- Dong, Y.; Mao, S.; Guo, F.; Shu, R.; Bai, J.; Qian, L.; Bai, Y. Coal Gasification Fine Slags: Investigation of the Potential as Both Microwave Adsorbers and Catalysts in Microwave-Induced Biomass Pyrolysis Applications. Energy 2022, 238, 121867. [Google Scholar] [CrossRef]
- Kumar Mishra, R. Pyrolysis of Low-Value Waste Switchgrass: Physicochemical Characterization, Kinetic Investigation, and Online Characterization of Hot Pyrolysis Vapours. Bioresour. Technol. 2022, 347, 126720. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Santamaria, L.; Amutio, M.; Artetxe, M.; Arregi, A.; Lopez, G.; Bilbao, J.; Olazar, M. Role of Temperature in the Biomass Steam Pyrolysis in a Conical Spouted Bed Reactor. Energy 2022, 238, 122053. [Google Scholar] [CrossRef]
- Duman, G.; Yanik, J. Two-Step Steam Pyrolysis of Biomass for Hydrogen Production. Int. J. Hydrogen Energy 2017, 42, 17000–17008. [Google Scholar] [CrossRef]
- Maisyarah, A.; Shiun, J.; Nasir, F.; Hashim, H. Ultimate and Proximate Analysis of Malaysia Pineapple Biomass from MD2 Cultivar for Biofuel Application. Chem. Eng. Trans. 2018, 63, 127–132. [Google Scholar] [CrossRef]
- Persson, H.; Yang, W. Catalytic Pyrolysis of Demineralized Lignocellulosic Biomass. Fuel 2019, 252, 200–209. [Google Scholar] [CrossRef]
- Palamanit, A.; Khongphakdi, P.; Tirawanichakul, Y.; Phusunti, N. Investigation of Yields and Qualities of Pyrolysis Products Obtained from Oil Palm Biomass Using an Agitated Bed Pyrolysis Reactor. Biofuel Res. J. 2019, 6, 1065–1079. [Google Scholar] [CrossRef]
- Jawad Kadhum, H.; Murthy, G.S. Novel System Design for High Solid Lignocellulosic Biomass Conversion. Bioresour. Technol. 2022, 350, 126897. [Google Scholar] [CrossRef]
- Tahir, M.H.; Çakman, G.; Goldfarb, J.L.; Topcu, Y.; Naqvi, S.R.; Ceylan, S. Demonstrating the Suitability of Canola Residue Biomass to Biofuel Conversion via Pyrolysis through Reaction Kinetics, Thermodynamics and Evolved Gas Analyses. Bioresour. Technol. 2019, 279, 67–73. [Google Scholar] [CrossRef]
- Mesfun, S.; Matsakas, L.; Rova, U.; Christakopoulos, P. Technoeconomic Assessment of Hybrid Organosolv-Steam Explosion Pretreatment of Woody Biomass. Energies 2019, 12, 4206. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. Effect of the Temperature on the Composition of Lignin Pyrolysis Products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Braz, W.C.N.C.E.M.; Ribeiro, S.A.C.A. Mixture of Biomass to Energy Reuse. J. Therm. Anal. Calorim. 2018, 131, 765–769. [Google Scholar] [CrossRef]
- Gil, M.V.; Oulego, P.; Casal, M.D.; Pevida, C.; Pis, J.J.; Rubiera, F. Bioresource Technology Mechanical Durability and Combustion Characteristics of Pellets from Biomass Blends. Bioresour. Technol. 2010, 101, 8859–8867. [Google Scholar] [CrossRef] [PubMed]
- Lajili, M.; Limousy, L.; Jeguirim, M. Physico-Chemical Properties and Thermal Degradation Characteristics of Agropellets from Olive Mill by-Products/Sawdust Blends. Fuel Process. Technol. 2014, 126, 215–221. [Google Scholar] [CrossRef]
- Boumanchar, I.; Chhiti, Y.; Ezzahrae, F.; Alaoui, M.; El, A.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Effect of Materials Mixture on the Higher Heating Value: Case of Biomass, Biochar and Municipal Solid Waste. Waste Manag. 2016, 61, 78–86. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Yan, W.; Vásquez, V.R.; Coronella, C.J. Hydrothermal Carbonization of Various Lignocellulosic Biomass. Biomass-Convers. Biorefinery 2014, 5, 173–181. [Google Scholar] [CrossRef]
- Pattiya, A. Fast Pyrolysis; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081010297. [Google Scholar]
- Di Blasi, C.; Branca, C.; D’Errico, G. Degradation Characteristics of Straw and Washed Straw. Thermochim. Acta 2000, 364, 133–142. [Google Scholar] [CrossRef]
- Carrier, M.; Neomagus, H.W.; Görgens, J.; Knoetze, J.H. Influence of Chemical Pretreatment on the Internal Structure and Reactivity of Pyrolysis Chars Produced from Sugar Cane Bagasse. Energy Fuels 2012, 26, 4497–4506. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, D.H.; Jae, J.; Lam, S.S.; Park, E.D.; Park, Y.K. Recent Advances in Catalytic Co-Pyrolysis of Biomass and Plastic Waste for the Production of Petroleum-like Hydrocarbons. Bioresour. Technol. 2020, 310, 123473. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A. Fast Pyrolysis of Biomass Thermally Pretreated by Torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 95–102. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Wang, X.; He, F.; Li, H. Effect of Torrefaction on Structure and Fast Pyrolysis Behavior of Corncobs. Bioresour. Technol. 2013, 128, 370–377. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR Studies of the Changes in Wood Chemistry Following Decay by Brown-Rot and White-Rot Fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Singh, D.; Zeng, J.; Laskar, D.D.; Deobald, L.; Hiscox, W.C.; Chen, S. Investigation of Wheat Straw Biodegradation by Phanerochaete Chrysosporium. Biomass Bioenergy 2011, 35, 1030–1040. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Y.; Ma, F.; Zhang, X.; Yu, H. Effect of Biopretreatment on Thermogravimetric and Chemical Characteristics of Corn Stover by Different White-Rot Fungi. Bioresour. Technol. 2010, 101, 5475–5479. [Google Scholar] [CrossRef]
- Charisteidis, I.; Lazaridis, P.; Fotopoulos, A.; Pachatouridou, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Triantafyllidis, K. Catalytic Fast Pyrolysis of Lignin Isolated by Hybrid Organosolv—Steam Explosion Pretreatment of Hardwood and Softwood Biomass for the Production of Phenolics and Aromatics. Catalysts 2019, 9, 935. [Google Scholar] [CrossRef]
- Li, X.; Su, L.; Wang, Y.; Yu, Y.; Wang, C.; Li, X.; Wang, Z. Catalytic Fast Pyrolysis of Kraft Lignin with HZSM-5 Zeolite for Producing Aromatic Hydrocarbons. Front. Environ. Sci. Eng. China 2012, 6, 295–303. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and Practice of Biomass Fast Pyrolysis Processes for Liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Bridge, S.A. A Review of Biomass Pyrolysis and Pyrolysis Technologies. In Biomass Pyrolysis Liquids Upgrading and Utilization; Springer: Berlin/Heidelberg, Germany, 1991; pp. 11–92. [Google Scholar] [CrossRef]
- Mamphweli, N.S.; Meyer, E.L. Implementation of the Biomass Gasification Project for Community Empowerment at Melani Village, Eastern Cape, South Africa. Renew. Energy 2009, 34, 2923–2927. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An Overview of Fast Pyrolysis of Biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Krishna, B.B.; Biswas, B.; Kumar, J.; Singh, R.; Bhaskar, T. Role of Reaction Temperature on Pyrolysis of Cotton Residue. Waste Biomass Valorization 2016, 7, 71–78. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of Temperature on Pyrolysis Products from Four Nut Shells. J. Anal. Appl. Pyrolysis 2006, 76, 285–289. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of Time and Temperature on Pyrolysis of Plastic Wastes in a Semi-Batch Reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Angin, D. Effect of Pyrolysis Temperature and Heating Rate on Biochar Obtained from Pyrolysis of Safflower Seed Press Cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis Polygeneration of Poplar Wood: Effect of Heating Rate and Pyrolysis Temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of Pyrolysis Temperature and Correlation Analysis on the Yield and Physicochemical Properties of Crop Residue Biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, N.; Ling, J.; Xu, C. A New Pyrolysis Technology and Equipment for Treatment of Municipal Household Garbage and Hospital Waste. Renew. Energy 2003, 28, 2383–2393. [Google Scholar] [CrossRef]
- Demirbaş, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Itoh, T.; Fujiwara, N.; Iwabuchi, K.; Narita, T.; Mendbayar, D.; Kamide, M.; Niwa, S.; Matsumi, Y. Effects of Pyrolysis Temperature and Feedstock Type on Particulate Matter Emission Characteristics during Biochar Combustion. Fuel Process. Technol. 2020, 204, 106408. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy, N.V.S.N.; Cortada, M.; Wang, L.; Hallett, J.; Shah, N.; Berkeley, L.; et al. The Multi-Scale Challenges of Biomass Fast Pyrolysis and Bio-Oil Upgrading: Review of the State of Art and Future Research Directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The Influence of Manure Feedstock, Slow Pyrolysis, and Hydrothermal Temperature on Manure Thermochemical and Combustion Properties. Waste Manag. 2019, 88, 85–95. [Google Scholar] [CrossRef]
- Acocke, G.V.C.; Dick, C.M.; Hague, R.A.; Cooke, L.A.; Bridgwater, A.V. Comparison of Ablative and Fluid Bed Fast Pyrolysis Products: Yields and Analyses. In Biomass for Energy and the Environment; Elsevier Ltd.: Amsterdam, The Netherlands, 1997; Volume 3, pp. 1632–1637. [Google Scholar]
- Zabaniotou, A.; Rovas, D.; Delivand, M.K.; Francavilla, M.; Libutti, A.; Cammerino, A.R.; Monteleone, M. Conceptual Vision of Bioenergy Sector Development in Mediterranean Regions Based on Decentralized Thermochemical Systems. Sustain. Energy Technol. Assess. 2017, 23, 33–47. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Biomass, B.T.G.; Group, T. Fast Pyrolysis Technology Development. Biofuels Bioprod. Biorefin. 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Świechowski, K.; Liszewski, M.; Babelewski, P.; Koziel, J.A.; Białowiec, A. Fuel Properties of Torrefied Biomass from Pruning of Oxytree. Data 2019, 4, 55. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of Temperature on Pyrolysis Products from Biomass. Energy Sources, Part A Recover. Util. Environ. Eff. 2007, 29, 329–336. [Google Scholar] [CrossRef]
- Xu, W.C.; Tomita, A. The Effects of Temperature and Residence Time on the Secondary Reactions of Volatiles from Coal Pyrolysis. Fuel Process. Technol. 1989, 21, 25–37. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic Fast Pyrolysis of Lignocellulosic Biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.G.; Brammer, J.G. Estimation of the Production Cost of Fast Pyrolysis Bio-Oil. Biomass Bioenergy 2012, 36, 208–217. [Google Scholar] [CrossRef]
- Wang, J.; Ku, X.; Lin, J.; Yang, S. Impact of the Reactor Structure on Biomass Pyrolysis in Fluidized-Bed Reactors: A Coarse-Grained CFD-DEM Study. Energy Fuels 2021, 35, 10035–10050. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. Pyrolysis of Biomass. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Academic Press: Cambridge, MA, USA, 2019; pp. 217–244. [Google Scholar] [CrossRef]
- Xu, B.; Li, A. Effect of High-Pressure on Pine Sawdust Pyrolysis: Products Distribution and Characteristics. In Proceedings of the International Conference on Green Energy and Sustainable Development (GESD 2017), Chongqing, China, 27–28 May 2017; Volume 1864, p. 020116. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. State of the Art of Applied Fast Pyrolysis of Lignocellulosic Materials a Review. Bioresour. Technol. 1999, 68, 71–77. [Google Scholar] [CrossRef]
- Fletcher, C. Computational Techniques for Fluid Dynamics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 1–46. [Google Scholar] [CrossRef]






| Aims/Objective | Methods | Ref. |
|---|---|---|
| Nine holocelluloses (two forestry and seven agricultural wastes) were selected as the feedstock to investigate the impact on the compositions of bio-oils and to screen the best feedstock suitable for the production of long-chain ethers precursor, for the ensuing improvement of yield and selectivity | Preparation of native holocellulose, evaluation of the sample, experimental apparatus, and procedures | [49] |
| To offer details on the yields and features of char produced from ten types of wood that are common in Southern Europe, undergoing biomass carbonization technologies condition | Biomass feedstocks, experimental facility, experimental procedure, charcoal characterization, and overview of the experiments | [50] |
| To perform intricate experimental analysis as well as the numerical modeling of oat straw’s slow pyrolysis. The pyrolysis products are described using advanced methods of analysis, with tests focusing on the properties and yield of the solid, liquid, and gaseous species | Feedstock sample, ultimate and proximate analyses and employing semi-batch vertical reactor where simultaneous thermal, infrared spectroscopy, qualitative of tars were analyzed, and pyrolysis gas analyzation, and numerical computations | [51] |
| The wet torrefaction of corn stalk was studied, and the biomass pyrolysis polygeneration performance of the wet torrefied sample was examined. More so, the solid material, energy, carbon, and hydrogen yields, as well as the effectiveness of removing ash and oxygen were also compared between WT and dry torrefied (DT) of corn stalks | Materials, torrefaction technique, characterization of torrefied samples, and pyrolysis technique | [52] |
| The determination of the thermal degradation characteristics of heating residues of eucalyptus (EU) and corncob (CC) for gasification using TGA rates of 10 °C/min in a nitrogen environment. The study covers the impact of biomass composition and kinetic parameters on heating rate | Preparation of biomass samples and experimental procedures | [32] |
| This experimental study set out to characterize the bioenergy potential of DS pyrolysis, measure gas emissions and byproducts, estimate kinetic and thermodynamic parameters, and detect the joint optimization of multiple responses in response to changing biofeedstock, heating rate, and temperature, as well as significant interactions between operational conditions | Sample preparation, physical and chemical analysis, TG experiments beforehand, activation energy, pyrolytic characteristic parameters, Friedman and Starink methods, Py-GC/MS experiments, TGA–FTIR experiments, and joint optimizations | [53] |
| To provide a thorough understanding of primary volatile compositions, mass loss behavior, reaction kinetics, and formation pathway during fast RH pyrolysis | Materials, pyrolysis process and kinetic methods | [31] |
| The impact of feedstock particle size on the distribution of fast pyrolysis products and the kinetics of slow pyrolysis | Characterization of MWSD, thermogravimetric analysis, evaluation of apparent activation energy, the pyrolysis of MWSD and product characterization, different profiles of mass loss and the impact of particle size on mass loss | [54] |
| To look into the reproducibility of TGA biomass pyrolysis experiments and potential deviations when mass loss kinetics are calculated from the same sample using various TGA technologies | TGA experiments and kinetic analysis | [33] |
| To fill the knowledge gap in orange and potatoes peel pyrolysis kinetics that was discovered during the literature review | Materials, TGA, and kinetics | [55] |
| To accurately evaluate the HHV using lumped-parameter pyrolysis kinetic models, and to demonstrate a straightforward correlation that can be used to assess HHV without relying on three different biomass species | Experimental samples, experimental procedures, and experimental results | [56] |
| Examine the combustion kinetics and study the combustion properties of five different types of biomass fuel pellets that can be used as biomass fuel | Analysis of the thermal weight loss and the components of five different biomass fuel pellet types | [44] |
| To investigate how the content of the biomass influences the kinetics, temporal evolution of the pyrolysis vapors, and production of the main bio-oil components during biomass pyrolysis | Materials, Py-FTIR analysis, isothermal mass loss of biomass, and using Py-GC/MS for the product analysis | [57] |
| To investigate the thermal decomposition of stalk and sour cherry flesh using thermogravimetric analysis, and to evaluate the activation energies using three kind of isoconversional approaches—Flynn–Wall–Ozawa, Friedman, or Kissinger–Akahira–Sunose. The findings reveal the pyrolysis kinetics and characteristics, as well as the ideal conditions for designing, optimizing, and simulating the pyrolysis process | Materials, physicochemical characterization, thermogravimetric analysis, and kinetic modeling | [58] |
| TGA/DTG investigation in an inert environment was performed to examine the thermal degrading and pyrolysis kinetics of biowastes. | Collection and preparation of biomass, proximate and ultimate investigation of samples as well as the calorific value, thermogravimetric/FTIR analysis | [59] |
| To carry out an extensive study that includes biochemical and physicochemical characterization, and the kinetic thermodynamic study of pyrolysis and thermal breakdown behavior of biomass from banana leaves | Sample preparation, banana leaves biomass pyrolysis reaction model determination using kinetic modeling, thermodynamic analysis, and thermogravimetric experiments | [60] |
| To clarify the pyrolytic behavior in terms of thermodynamic and kinetic characteristics, as well as the bioenergy potential of biological wastes resulting from the manufacturing of bio-products | The processing of bacterial biomass produced in a pilot-scale operation, sample characterization, FTIR spectroscopy, data processing using PCA, a TGA experiment, the characteristics of pyrolysis, thermo-kinetic studies pyrolysis, Py-GC/MS analysis, and the development of a model based on SVR | [61] |
| Pyrolyze three samples using thermogravimetric analysis and characterize them by determining how well various Phragmites Hirsuta components pyrolyze, thus this study offers theoretical direction for the formulation of the Phragmites preparation process, bioenergy is converted into Hirsuta by a thermochemical process | Material, characterization, Thermogravimetric analysis, kinetic modeling, reaction model determination, and thermodynamic analysis | [62] |
| To outline a straightforward method for analyzing the kinetic parameters (frequency factor, activation energy, and reaction model) of biomass with complicated thermal behavior. A multi-step mechanism for the biomass pyrolysis processes was employed to get the kinetic parameters using a deconvolution algorithm process coupled with isoconversional approaches. | Sample selection, preparation, and characterization, performed kinetics, and thermogravimetric analysis | [63] |
| In-depth research was conducted on the mechanisms causing the variations and the correlations between the pyrolysis characteristics and the various types of biomass. By improving our knowledge of the pyrolysis process in various biomass types, this work also serves as a reference for their thermal conversion methods | Materials, physicochemical of biomass, thermogravimetric, and kinetic analysis using the Coats–Redfern method TG and multi-peak fitting in the derivative thermogravimetric analysis. | [64] |
| Using a laboratory-scale (5 kg/h) AFP unit to accurately assess the impact of feedstock type on the characteristics of bio-oils produced from straw, miscanthus, and beech and poplar wood | Biomass that has been pyrolyzed, the pyrolysis process, the physicochemical characteristics of bio-oils, and a quantitative analysis of the chemical makeup of bio-oils | [6] |
| On the physical and chemical characteristics of biochar, particularly their effects on nitrogen (N) content and composition, the impact of feedstock type and temperature of pyrolysis were examined | Materials, preparation of biochar and sample preparation, and analytical methods | [65] |
| Studies involving feedstock, pyrolysis, and biochar, including policies on emission | Reviewing different concepts | [66] |
| The investigation of the effects of CaO addition sorbent and the temperature of pyrolysis on the chemical and the physical characteristics of obtained biochar and syngas | Material characteristics, experimental procedure, and methods | [38] |
| To look into how the structure of the resulting bio-char changed as the gaseous and liquid products evolved in relation to the pyrolysis temperature, and understanding how temperature affects the development of organics and the composition of biochar | Feedstock and chemicals, pyrolysis experiments, characterization of the products, and kinetic analysis | [43] |
| To ascertain how the duration time and pyrolysis temperature affect the properties of hydrochars in comparison to biochars produced through direct slow pyrolysis. In order to do this, hydrochar produced by HTC of waste biomass was pyrolyzed at two different temperatures (350 and 500 °C) and three different times (1, 3 and 5 h), and the testing was conducted to establish a number of properties relevant to the use of chars as soil amendment, inexpensive adsorbent, or fuel, and growing media, including pH, electrical conductivity, electrochemical potential, porosity, phytotoxicity, and elemental composition | Selection of hydrochar, pyrolysis of hydrochar made from waste biomass, pyrolysis of waste biomass, and char characterization | [67] |
| To investigate the impact of the pyrolysis temperature using fluidized bed pyrolysis system, three reactions were carried out to convert solid waste into renewable aviation fuel in attempt to show the distributions of the liquid and gas products at different temperatures | Feedstock, equipment, experimental procedures, and product analysis | [26] |
| The reaction mechanism of the co-pyrolysis of biomass and coal in the TGA analyzer was investigated using both conventional TGA and a novel congruent-mass TGA analyses. Studies that compare how these two approaches differ in how they assess the likelihood of a coal–biomass interaction | Materials and TGA | [47] |
| To research the kinetics of the co-pyrolysis of the coal and pretreated watermelon rind (WMR) blends | Selection of the biomass, pretreatment, compositional analysis, determination of the (WMR) higher heating value, calculation of its exergy, preparation of sample blends, thermogravimetric analysis of the coal and pretreated (WMR), kinetic analysis, and estimation of the thermodynamic parameters | [68] |
| The following research goals were achieved: (a) performing a thorough thermogravimetric analysis (TGA) of the nut shells; (b) identifying the characteristic points in the nut shells’ thermal decomposition process; (c) determining the temperature range at which hemicellulose, cellulose, and lignin decomposed in the examined nut shells; (d) estimating the fundamental kinetic parameters of the nut shells thermal decomposition; and (e) the physiochemical properties of the nut shells conversion rates as a function of the process temperature | Characteristics of the feedstock used in the research, thermogravimetric analysis, kinetic modelling, and model-fitting method: Coats–Redfern Method | [42] |
| TGA–FTIR (thermogravimetric analysis with FTIR analysis of evolved gases) pyrolysis experiment combined with advanced data analysis and modeling methods to assess the viability of developing an advanced methodology for the evaluation of biomass materials | Selection of the sample and testing on a suite of biomass materials | [41] |
| To assess the pyrolysis behavior of corks with various properties that might be used in scaling up the pyrolysis of cork-rich materials, in the strengthening of their value as well as their integration in thermochemical platforms | Materials, thermogravimetric analysis, kinetic analysis, estimation of chemical composition, wet chemical characterization, and FTIR analysis | [69] |
| The characteristics of green corn husks were described and analyzed in order to determine the thermokinetics conversion parameters through pyrolysis reactions that were kinetically studied using TGA and DTG, where the Flynn–Wall–Ozawa was used to compare the energetic efficiency from corn husk | Materials, biomass composition analysis, higher calorific value, non-isothermal thermogravimetric analyses, thermokinetics studies, master plots method, kinetic model proposed by Kissinger, kinetic model of Friedman, thermogravimetric analysis, and the mathematical simulation of the thermal decomposition kinetic of green corn husk biomass | [70] |
| To look into the technical and financial effects of different lignocellulosic elements on biomass pyrolysis, this work specifically investigates the basic mechanisms of cellulose, hemicellulose, and lignin transformation during pyrolysis | Characterization of biomass samples, sample preparation, pyrolysis, economic analysis, and validation via experimental values | [71] |
| To make available a theoretic framework for advancing the pyrolysis process and the efficient use of corn straw resources | Experimental materials, Instruments, and methods, analytical methods, and kinetics theory | [72] |
| Utilizing the pyrolysis poly-generation method to provide renewable energy and materials while overcoming the drawbacks of using rice husks | Materials, the preparation of an activated bio-char catalyst, a catalytic fast pyrolysis process, derived of amorphous SiO2 and porous carbon from bio-char, experiments on the adsorption of organic compounds, and physicochemical analysis | [34] |
| To research, ascertain, and comprehend these solids’ digestibility, as well as how the various hybrid method process parameters affected it | Feedstock and inoculum, pretreatment of wood chips, anaerobic digestion of pretreated solids and other analytical methods | [35] |
| In light of the fantastic outcomes produced in the chemical activation of rice husks (RHs), an assessment of bio-char made from RH pyrolysis was conducted to see if it could be used as a solid-phase extraction (SPE) to filter out harmful organic compounds from the biooil aqueous phase | Pyrolysis, chemical activation, characterization of activated carbon, SPE procedures, HPLC-DAD analysis, and method validation | [37] |
| Researchers have looked into the non-catalytic and catalytic co-pyrolysis of Ulva prolifera macroalgae (UPM) and straw (RS). To establish their ideal values, it has been investigated how temperature and mixing ratio affect the product’s distribution | Feedstock characterization, experimental setup and procedures, catalysts preparation, catalyst characterization methods, and liquid products analysis methods | [39] |
| Studies of techno-economic performance of involving biorefinery concepts and steam pretreatment techniques | Feedstock composition/economic analysis | [73] |
| Based on the composition of the ash, the investigation’s goal was to pinpoint the pertinent fractionation processes; the findings will later be applied to create a model for predicting slag composition and viscosity based on process parameters and fuel ash composition | Materials, feedstock preparation, and gasification process, and product char and gas analysis | [74] |
| To create the biofuel using a variety of techniques and examine the fuel’s characteristics | Pyrolysis, extraction of pyrolysis oil, gasification, and procedure for producer gas generation, the analysis of the coconut shell using TGA, ultimate analysis, producer gas composition, and proximate analysis | [45] |
| A comparative investigation on the two-step pyrolysis (TSP) of lignocellulosic biomass was carried out on samples of walnut shell (WS), cotton stalk (CS), corncob (CC), and their acid-washed counterparts using TGA–FTIR and Py-GC/MS | Materials and preparation, samples characterizations, and TGA–FTIR and Py-GC/MS analysis | [40] |
| To assess how relations between lignin and cellulose, which occur during the co-pyrolysis of lignin and cellulose at temperatures between 100 and 350 °C, affect char structure changes | Sample preparation, fast pyrolysis experiments, and sample characterization | [75] |
| To examine the viability of spent coffee grounds (SCG) upcycling via pyrolysis for the production of biochar and energy, while also proposing a circular economy scenario for the effective use of SGC produced in the city of Larisa, Greece | Materials characteristics, pyrolysis and process protocol | [48] |
| To determine the levoglucosan percentage in the bio-oils prepared from fast pyrolysis of hydrochloric acid-treated and untreated rice husks (RHs) under vacuum conditions | Materials, characterization of RHs, pretreatment of RHs, Fast pyrolysis procedure, bio-oil characterization, and quantification of levoglucosan in bio-oils | [36] |
| To research the impact of total pressure, pyrolysis temperature, and CO2 concentration on biomass char gasification at various temperatures | Biomass samples, char preparation, and char reaction models | [76] |
| To research (a) the influence of biochar made from mesquite on the combined physical and hydraulic properties of various compacted soils, and (b) the interdependence of hydraulic properties of biochar-amended soil on the physical properties for possible use in bioengineered structures | Biochar, soils, physical properties, hydraulic properties, FTIR, FESEM, XRD, BET, and statistical analysis | [77] |
| To investigate levoglucosenone (LGO) production used levoglucosan (LGA) as feedstock. LGA dehydration has a lower activation energy and is chemically simpler than cellulose pyrolysis, enabling the reaction to occur at low temperatures | Materials, reaction, and product analysis | [11] |
| To look into how pressure affects the pyrolysis of biomass’s thermal effects. Corn stalks, popular, switchgrass Trail-blazer, and switchgrass Alamo were the four energy crops chosen for experimental characterization | Materials, experimental techniques, and procedures | [78] |
| To assess the physicochemical potential of palm waste for pyrolysis processes that result in the production of biofuels | Preparation of biomass samples, and determination of physicochemical properties | [79] |
| To clarify differences and similarities among the combustion of the original raw biowaste and the combustion of bio-oil and biochar in order to better understand how fly ash forms during these processes | Biomass, biochar and bio-oil, fuel preparation prior to combustion experiments, combustion experiments, particle sampling system, operational procedure, and experimental plan, chemical analysis of the particulate matter, and multivariate data analysis are all covered in this study | [80] |
| To look into the possibility of preventing agglomeration and enhancing sugar formation during the pyrolysis of herbaceous biomass by combining ferrous, magnesium, and ammonium cations with sulfate anions | Methods for pretreatment, controlled pyrolysis duration-quench, continuous pyrolysis reactor system, assessment of sustainable throughput, quantification of sugar, ICP digestion, scanning, and electron microscopy analysis | [81] |
| To research the energy potential of hydrochar made from straw, Virginia mallow, and wood (pine) biomass. The hydrochars’ pyrolysis process was therefore investigated in order to determine how the gaseous byproducts changed with pyrolysis temperature | Materials, hydrothermal carbonization process, and pyrolysis | [82] |
| As an alternative technique for using waste biomass in the Polish context, a thorough study of slow solar pyrolysis of various waste biomass feedstock is presented. Although slow solar pyrolysis is the least expensive technology available due to the low heat input, it has the potential to produce highly porous solid fuels and provide a long-term solution for difficult waste disposal | Feedstock characterization includes determining the amount of lignocellulose in the feedstock as well as its ultimate and proximate analyses.sample preparation, sample analysis for C, H, and N, and BET surface area measurement of porosity | [83] |
| In order to comprehend pyrolysis behavior and potential interactions, investigations into the thermal decomposition of lignin and lignocellulosic biomass (watermelon rind) WMR were carried out at 325–625 °C to pyrolyze various lignin components in order to improve the pyrolytic products | Materials, experimental set-up and procedures, and product analysis | [84] |
| Experimental Objectives | Pre-Pyrolysis | Main-Pyrolysis | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Biomass Selection | Analytical Method | Biomass Treatment Method | Reactor Types | Operating Process | Product Output | |||
| One or More | Biomass Type | Moisture, Organic Matter, Ash Content, and Others | ||||||
| To look into the yield and characteristics of the pyrolysis reaction products made from palm oil (trunk, frond, and shell) in an agitated reactor | Palm (trunk, frond and shell) | Palm tree | Moisture, ash content, and others | Physical and thermal | Agitated pyrolysis reactor, TGA, and DTA | Pyrolysis | Gas, bio-oil, and char | [103] |
| To investigate the influence of pyrolysis temperature (500–800 °C) on product yields in a conical spouted bed reactor with steam as a fluidizing source | Pine wood sawdust | Wood | Moisture, ash content, and others | Physical and thermal | Conical spouted bed | Pyrolysis | Gas, bio-oil, and char | [99] |
| Using steam pyrolysis of olive pomace, it was investigated how well various char-based catalysts (including biochar and coal char) produced hydrogen | Olive pomace | Olive | Moisture, ash content, and others | Physical and thermal | Fixed bed, TGA and others | Pyrolysis | Gas, bio-oil, char, and hydrogen | [100] |
| It was investigated how well the wet torrefied sample performed in the biomass pyrolysis polygeneration process as well as the WT of corn stalk | Corn stalk | Corn | Moisture, ash content, and others | Physical and thermal | Fixed bed | Pyrolysis | Biochar | [52] |
| Based on the characteristics of the pyrolysis process and its effectiveness in catalytic upgrading, the catalytic and non-catalytic pyrolysis of demineralized biowaste was examined and compared to raw biomass | Sawdust | Softwood | Moisture, ash content, and others | Chemical, physical and thermal | Fixed bed, Py-GC/MS, | Pyrolysis | Gas, bio-oil, and char | [102] |
| Examining the energetic, physical, and chemical characteristics of various biomass feedstocks in order to characterize their performances | Grapevine, olive trees, and others | Lignocellulosic residues | Moisture, ash content, and others | Physical and thermal | TGA | Pyrolysis | Bio-char and bio-fuel | [89] |
| To successfully scale up the pyrolysis process, it is crucial to thoroughly understand the effects of key variables on the devolatilization kinetics and bio-oil composition, such as biomass particle size, shape, content, heating rate, and residence period. | Saw dust | Wood | Moisture, ash content, and others | Physical and thermal | Pyroprobe® 5200 | Pyrolysis | Biochar and bio-oil | [54] |
| To ascertain the thermodynamic parameters and the kinetic triplet (activation energy, pre-exponential variable, and reaction model) | Banana leaves | Banana | [60] | |||||
| Devoted to researching the online characterization, kinetic and thermodynamic analysis, thermal decomposition, and physicochemical characterization of hot vapors released during pyrolysis | Switchgrass | Crop | Moisture, ash content, and others | Physical and thermal | TGA-FTIR, Py-GC–MS examination | Pyrolysis | Gas, bio-oil, and char | [98] |
| This study looks at the effects of CaO on the evolution properties of cellulose, hemicellulose, and lignin pyrolysis products using TGA–FTIR and Py-GC/MS, and it also discusses the reaction mechanism of CaO-assisted pyrolysis of biowaste components | Cellulose and beechwood | Mixed | Moisture, ash content, and others | Chemical, physical and thermal | TGA–FTIR and PY-GC/MS | Pyrolysis | Bio-oil from | [25] |
| Using slow pyrolysis in a thermogravimetric analyzer, investigate the decomposition mechanism of the lab-scale grown microalga | Algal biomass | Algal | Moisture, ash content, and others | Physical and thermal | TGA | Pyrolysis | Biochar | [92] |
| (a) To methodically examine the recovery effectiveness of reducing sugars and VFAs at various HTS (4.17–8.28, 190–320 °C), and (b) to characterize the structure of the cornstalk following hydrothermal treatment at various HTS | Cornstalk | Corn | Moisture, ash content, and others | Physical | Batch | HTC | Volatile fatty acids (VFAs) and sugars | [88] |
| Determine the entrained flow reactor (EFR) used for the beech wood pyrolysis experiments, which were conducted at various gas residence times with temperature between 500 and 1400 °C. These experimental conditions were broad enough to produce chars with a range of characteristics | Beech | Wood | Moisture, ash content, and others | Physical and thermal | Entrained flow | Pyrolysis | Biochar | [15] |
| To investigate the characteristics of MD2 pineapple waste and its potential to become a feedstock for alternative solid biofuel | Pineapple | Pineapple | Moisture, fixed carbon content, and others | Physical and thermal | TGA | Pyrolysis | Biochar | [101] |
| To illustrate how canola residue may be a suitable biofuel feedstock for low-temperature (<450 °C) slow pyrolysis with energetically favorable conversions of up to 70 wt.% of volatile matter | Canola residue | Canola | Moisture, ash content, and others | Physical and thermal | TGA–FTIR | Slow pyrolysis | Bio-fuel | [105] |
| (1) To determine the transformation behavior of HMs during co-HTC, and (2) to investigate the fuel properties of the hydrochar from co-HTC. The results could provide support for SS utilization, particularly for fuel production with the targeted regulation of HMs | Sludge and biomass | Sludge and lignocellulosic | Moisture, ash content, and others | Physical | Autoclave reactor | HTC | Liquid and hydrochar | [90] |
| HTL thermal transformation of tobacco industry biowaste to oil in a multiple batch reactor | Tobacco | Tobacco | - | Physical | Batch reactor | HTL | Biocrude | [91] |
| Having in mind the literature presented on solar pyrolysis so far, a thorough study on slow solar pyrolysis of various waste biomass feedstocks is presented as an alternative method for using waste biomass in the Polish scenario, with a primary focus on fast and flash pyrolysis | Wood, stray sewage sludge | Mixed | Moisture, ash content, and others | Physical and thermal | Fixed-bed, TGA, and others | Pyrolysis | Gas, bio-oil, and char | [83] |
| To thoroughly investigate the catalytic potential of NZ (commonly found in Pakistan) in comparison to that of commercial ZSM-5 for raw and pretreated rice straw | Rice straw | Rice | Moisture, ash content, and others | Physical, chemical, and thermal | Fixed-bed | Pyrolysis | Gas and bio-oil | [93] |
| By combining acid impregnation and two-staged pyrolysis, the study aims to achieve staged and directional valorization of holocellulose and lignin in biomass waste | Eucalyptus waste | Wood | Moisture, ash content, and others | Physical, chemical, and thermal | Torrefaction and fast pyrolysis | Char, anhydrosugars, and phenols | [94] | |
| In order to maximize utilization, it is important to compare specifically how well two common agricultural and forestry biomasses are suited for bioenergy production | Rice husk and poplar bark | Rice and wood | Moisture, ash content, and others | Physical and thermal | TG/DTG | Pyrolysis | Biochar | [95] |
| In particular, the effects on nitrogen (N) content and composition were examined, along with the impact of biomass type and pyrolysis temperature on the physical and chemical properties of biochar | Soybean straw and chlorella | Crop type | Moisture, ash content, and others | Physical and thermal | Stainless steel cylinder and electric muffle furnace | HTC/pyrolysis | Hydrochar and biochar | [65] |
| Study to lower energy consumption and increase glucose concentrations in enzymatic hydrolysis reactors | Wheat straw | Wheat | - | Chemical | Hydrolysis reactor | Hydrolysis and fermentation | Glucose | [104] |
| In the work, the catalytic activity of supported Al-containing bimetals was studied, and the synergy between the bimetals was discussed. In addition, the reaction pathways on the formation of furans were proposed | Corncob, wood, and others | Mixed | Moisture, ash content, and others | Physical and thermal | Py-GC × GC/MS | Pyrolysis | Furan | [96] |
| To research the microwave heating properties of coal gasification fine slag and its pyrolysis of biomass catalytic properties | Pine sawdust | Wood | Moisture, ash content, and others | Physical and thermal | Quartz tube, microwave-induced | Pyrolysis and gasification | Gas, bio-oil, and char | [97] |
| Reactor/Analytical Tools | Biomass/Feedstock | Ref. | ||
|---|---|---|---|---|
| Mode | Types | Group Name | Group Examples | |
| Single | TGA | Woody | Eucalyptus | [32] |
| Woody | Pellet | [44] | ||
| Corn | Straw | [72] | ||
| Walnut | Nut shell | [42] | ||
| Hazelnut | Nut shell | [42] | ||
| Pistachio | Nut shell | [42] | ||
| Cork species | Cork | [69] | ||
| Sugarcane | Bagasse | [63] | ||
| Corn | Husk | [70] | ||
| Wheat | Straw | [47] | ||
| Woody | Bamboo | [47] | ||
| Rice | Husk | [31] | ||
| Woody | beech | [33] | ||
| Peanut | Straw | [64] | ||
| Sesame | Stalk | [64] | ||
| Rape | Pod | [64] | ||
| Tobacco | Stem | [64] | ||
| Pecan | Shell | [64] | ||
| Bada wood | Shell | [64] | ||
| Woody | Camphor Tree | [64] | ||
| Woody | Sapele | [64] | ||
| Peanut | Straw | [64] | ||
| Sesame | Stalk | [64] | ||
| Woody | Poplar | [64] | ||
| Woody | Willow | [64] | ||
| Sour cherry | Stalk | [41] | ||
| Sour cherry | Flesh | [41] | ||
| Phragmites hirsuta | Root | [62] | ||
| Phragmites hirsuta | Stem | [62] | ||
| Phragmites hirsuta | Leaves | [62] | ||
| Fixed bed | Rice | Husk | [34] | |
| Corn | Stalk | [34] | ||
| Oak | Cork | [50] | ||
| Oak | Holm | [50] | ||
| Wood | Waste wood | [83] | ||
| Herbaceous | Waste straw | [83] | ||
| Sewage sludge | Sludge | [83] | ||
| Woody | Anhydro sugar | [11] | ||
| Model compounds | Cellulose | [43] | ||
| Woody | Kraft | [75] | ||
| Alkali | [75] | |||
| Avicel | [75] | |||
| Ablative | Woody | Poplar | [6] | |
| Straw | [6] | |||
| Miscanthus | [6] | |||
| Fluidized bed | Herbaceous | Corn stover | [81] | |
| Rice husk | [26] | |||
| Entrained flow | Straw | Straw | [74] | |
| Furnace | Rice | Straw | [39] | |
| Vival prolifera macroalgae | Vival prolifera macroalgae | [39] | ||
| Wood | Shavings | [66] | ||
| Tubular quartz | Rice | Husk | [36] | |
| Adiabatic oxygen bomb calorimeter | Watermelon | Ring | [68] | |
| HTC | Parks | Park | [67] | |
| Gardens | Garden | [67] | ||
| Wire mesh | Sigma-Aldrich | Sigma-Aldrich | [71] | |
| Semi-batch vertical | Oat wood | Straw | [51] | |
| DTG | Woody | Japanese cedar | [56] | |
| Woody | Castanopsis | [56] | ||
| Rice | Straw | [56] | ||
| Rotary-klin prototype | Plant | Coffee plant | [48] | |
| Furnace | Woody | Stem | [80] | |
| Woody | Bark | [80] | ||
| Combined | TGA and DSC | Corn | Stalk | [78] |
| Switchgrass alamo | Grass | [78] | ||
| Woody | Poplar | [78] | ||
| TGA and STA | Banana | Leaves | [60] | |
| Pyro-Probe and CDS | Energy crop | Virginia mallow | [82] | |
| Woody | Pine | [82] | ||
| Grass | Straw | [82] | ||
| Gasifier and cylindrical reactor | Coconut | Shell | [45] | |
| TGA-FTIR and Py-Gc/MS | Corn | Cob | [40] | |
| Cotton | Stalk | [40] | ||
| Walnut | Shell | [40] | ||
| TGA and DTG | Orange | Peels | [55] | |
| Potato | Peels | [55] | ||
| Coffee | Husk | [59] | ||
| Coffee | Residue | [59] | ||
| Palm oil tree | Fronds | [79] | ||
| Shells | [79] | |||
| Roots | [79] | |||
| Trunk | [79] | |||
| Fixed bed and quartz | Rice | Husk | [37] | |
| TGA, DTG and fixed bed | Leaves | Birch | [73] | |
| Wood | Spruce | [73] | ||
| TGA and CDS | Wood | Sawdust | [54] | |
| Herbaceous | Corncob | [49] | ||
| Wheat straw | [49] | |||
| Rice husk | [49] | |||
| Py-FTIR and Pyro-probe | Rice | Husk | [57] | |
| Woody | Pine | [57] | ||
| Fruit | Bunch | [57] | ||
| TGA and Py-GC/MS | Durian | Shells | [53] | |
| TGA-FTIR | Woody | Populus deltoides | [41] | |
| Pinus radiata | [41] | |||
| Willow chips | [41] | |||
| Roasted cashew nut | [41] | |||
| Shells | Almond | [41] | ||
| Hazelnut | [41] | |||
| Brazil-nut | [41] | |||
| Roasted cashew nut | [41] | |||
| TGA–FTIR | Herbaceous | Reed canary grass | [41] | |
| Miscanthus giganteus | [41] | |||
| Spinach | [41] | |||
| Animal Product | Chicken manure | [41] | ||
| Model compounds | cellulose (avicel) | [41] | ||
| ALC lignin | [41] | |||
| Xylan | [41] | |||
| Dglucose | [41] | |||
| Pectin | [41] | |||
| Chlorogenic acid | [41] | |||
| TGA and furnace | Herbaceous | Pine | [76] | |
| Reactor Type | Technology Readiness | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Bubbling fluidized bed | Commercialized | Simplicity and ease to operation; efficient heat transfer; high bio-oil yield of 70–75% | Fine feedstock particles require | [127] |
| Circulating fluidized bed | Commercialized | suitable heat transfer, simpler scaling, and a useable particle size of 6 mm | More complex to operate and less liquid yield to achieve | [127,135] |
| Vacuum | Scaled up to about 3000 kg/h | No gas carrier is necessary, there are no complicated operating conditions, and it is possible to employ bigger biomass particles | Liquid yield (35–50%); large process equipment; slow heat transfer rate; greater coal content | [135] |
| Vortex | NREL | Particle sizes up to 20 mm, biomass particles were accelerated with high velocity, and yields of 65% liquids | High entering velocities of material into the reactor led to erosion at the transition from linear to angular momentum | [85] |
| Ablative | Laboratory scale | Larger particles may be used; there is no need for inert gas; heat transfer through hot reactor wall | Limitation on scale-up and heat supply issue | [138] |
| Auger | Pilot-scale, Understudy | Ceramic or still ball; sand as the heat carrier; mechanically driven | Bigger particles can be used; lesser liquid yield | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasaq, W.A.; Okpala, C.O.R.; Igwegbe, C.A.; Białowiec, A. Navigating Pyrolysis Implementation—A Tutorial Review on Consideration Factors and Thermochemical Operating Methods for Biomass Conversion. Materials 2024, 17, 725. https://doi.org/10.3390/ma17030725
Rasaq WA, Okpala COR, Igwegbe CA, Białowiec A. Navigating Pyrolysis Implementation—A Tutorial Review on Consideration Factors and Thermochemical Operating Methods for Biomass Conversion. Materials. 2024; 17(3):725. https://doi.org/10.3390/ma17030725
Chicago/Turabian StyleRasaq, Waheed A., Charles Odilichukwu R. Okpala, Chinenye Adaobi Igwegbe, and Andrzej Białowiec. 2024. "Navigating Pyrolysis Implementation—A Tutorial Review on Consideration Factors and Thermochemical Operating Methods for Biomass Conversion" Materials 17, no. 3: 725. https://doi.org/10.3390/ma17030725