In Vitro Release and In Vivo Pharmacokinetics of Praziquantel Loaded in Different Polymer Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer Particles Loaded with PZQ
2.2.1. PMMA Nanoparticles
2.2.2. PMMA Microparticles
2.2.3. Smart PMMA-co-DEAEMA and PMMA-co-DMAEMA Microparticles
2.3. Characterizations
3. Results and Discussion
3.1. Particle Size Distributions
3.2. Entrapment Efficiency
3.3. Dissolution
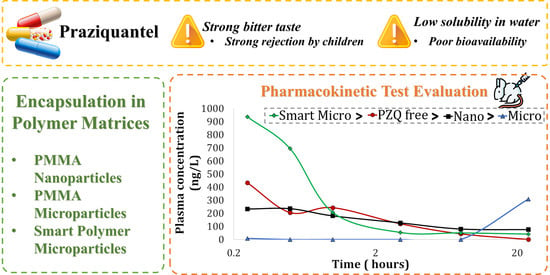
3.4. Pharmacokinetic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Connell, D. Neglected Diseases. Nature 2007, 449, 157. [Google Scholar] [CrossRef]
- WHO Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 2 February 2023).
- Pica-Mattoccia, L.; Cioli, D. Sex- and Stage-Related Sensitivity of Schistosoma Mansoni to in Vivo and in Vitro Praziquantel Treatment. Int. J. Parasitol. 2004, 34, 527–533. [Google Scholar] [CrossRef]
- da Paixão Siqueira, L.; Fontes, D.A.F.; Aguilera, C.S.B.; Timóteo, T.R.R.; Ângelos, M.A.; Silva, L.C.P.B.B.; de Melo, C.G.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Schistosomiasis: Drugs Used and Treatment Strategies. Acta Trop. 2017, 176, 179–187. [Google Scholar] [CrossRef]
- Costa, E.D.; Priotti, J.; Orlandi, S.; Leonardi, D.; Lamas, M.C.; Nunes, T.G.; Diogo, H.P.; Salomon, C.J.; Ferreira, M.J. Unexpected Solvent Impact in the Crystallinity of Praziquantel/Poly(Vinylpyrrolidone) Formulations. A Solubility, DSC and Solid-State NMR Study. Int. J. Pharm. 2016, 511, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Münster, M. Paediatric Drug Development for Praziquantel; Heinrich-Heine-Universität Düsseldorf: Düsseldorf, Germany, 2018. [Google Scholar]
- Mengarda, A.C.; Iles, B.; Longo, J.P.F.; de Moraes, J. Recent Trends in Praziquantel Nanoformulations for Helminthiasis Treatment. Expert. Opin. Drug. Deliv. 2022, 19, 383–393. [Google Scholar] [CrossRef]
- Al-kasmi, B.; Alsirawan, M.B.; Bashimam, M.; El-zein, H. Mechanical Microencapsulation: The Best Technique in Taste Masking for the Manufacturing Scale—Effect of Polymer Encapsulation on Drug Targeting. J. Control. Release 2017, 260, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material Encapsulation in Poly(Methyl Methacrylate) Shell: A Review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Akanksha Kumari, A.; Mishra, N. Polymers in Drug Delivery. J. Biosci. Med. 2016, 4, 69–84. [Google Scholar] [CrossRef]
- Tihan, T.G.; Ionita, M.D.; Popescu, R.G.; Iordachescu, D. Effect of Hydrophilic–Hydrophobic Balance on Biocompatibility of Poly(Methyl Methacrylate) (PMMA)–Hydroxyapatite (HA) Composites. Mater. Chem. Phys. 2009, 118, 265–269. [Google Scholar] [CrossRef]
- Awad, R.; Avital, A.; Sosnik, A. Polymeric Nanocarriers for Nose-to-Brain Drug Delivery in Neurodegenerative Diseases and Neurodevelopmental Disorders. Acta Pharm. Sin. B 2022, in press. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Liang, J.; Yuan, D.; Zhao, W. Research Progress of Poly(Methyl Methacrylate) Microspheres: Preparation, Functionalization and Application. Eur. Polym. J. 2022, 175, 111379. [Google Scholar] [CrossRef]
- Castor, C.A., Jr.; Azevedo, G.; Santos, J.G.F., Jr.; Pinto, J.C.; Pires, L.; Nele, M.; Pinto, M.; Paiva, T. PMMA-Based Biomedical Applications: Manufacture and Uses. In Advances in Medicine and Biology; Nova Medicine & Health: New York, NY, USA, 2019; Volume 141, pp. 97–167. ISBN 978-1-5361-5637-9. [Google Scholar]
- Bettencourt, A.; Almeida, A.J. Poly(Methyl Methacrylate) Particulate Carriers in Drug Delivery. J. Microencapsul. 2012, 29, 353–367. [Google Scholar] [CrossRef]
- Fonseca, L.B.; Nele, M.; Volpato, N.M.; Seiceira, R.C.; Pinto, J.C. Production of PMMA Nanoparticles Loaded with Praziquantel Through “In Situ” Miniemulsion Polymerization. Macromol. React. Eng. 2013, 7, 54–63. [Google Scholar] [CrossRef]
- da Fonseca, L.B.; Mattos, A.C.A.; Coelho, P.M.Z.; Araújo, N.; da Silva Zamith, H.P.; Volpato, N.M.; Nele, M.; da Silva Pinto, J.C.C. Desenvolvimento de Um Medicamento Brasileiro Nanoencapsulado Para o Tratamento Da Esquistossomose. Vigilância Sanitária Em Debate Soc. Ciênc. Tecnol. 2013, 1, 85–91. [Google Scholar] [CrossRef]
- Paiva, T.; Vieira, L.; Melo, P.; Nele, M.; Pinto, J.C. In Situ Incorporation of Praziquantel in Polymer Microparticles through Suspension Polymerization for Treatment of Schistosomiasis. Macromol. React. Eng. 2018, 13, 1800064. [Google Scholar] [CrossRef]
- Paiva, T.F.; Alves, J.B.; Melo, P.A.; Pinto, J.C. Development of Smart Polymer Microparticles through Suspension Polymerization for Treatment of Schistosomiasis. Macromol. React. Eng. 2019, 13, 1900028. [Google Scholar] [CrossRef]
- Alves, J.B.; Franckini Paiva, T.; Salim, V.M.; Conceição Ferraz, H.; Pinto, J.C. In Situ Encapsulation of Praziquantel through Methyl Methacrylate/Diethylaminoethyl Methacrylate and MMA/DMAEMA Miniemulsion Copolymerizations in Presence of Distinct Ionic Surfactants. SPE Polym. 2021, 2, 110–121. [Google Scholar] [CrossRef]
- Bratek-Skicki, A. Towards a New Class of Stimuli-Responsive Polymer-Based Materials—Recent Advances and Challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.-D.; Wang, Z.-C.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Synthesis and Properties of PH and Temperature Sensitive P(NIPAAm-Co-DMAEMA) Hydrogels. Colloids Surf. B Biointerfaces 2008, 64, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, O.L.; Syed Zameer Ahmed, K.; Sujatha, K.; Ponnmurugan, P.; Srivastava, A.; Ramesh, R.; Sukumar, R.; Elanithi, K. Fabrication and Characterization of Chicken Feather Keratin/Polysaccharides Blended Polymer Coated Nonwoven Dressing Materials for Wound Healing Applications. Mater. Sci. Eng. C 2018, 92, 26–33. [Google Scholar] [CrossRef]
- Nagappa, A.; Pandi, P. Effect of Acute and Chronic Treatment of Losartan Potassium on Tail-Flick Response in Mice. Indian J. Pharmacol. 2006, 38, 281. [Google Scholar] [CrossRef]
- Bardal, S.K.; Waechter, J.E.; Martin, D.S. Pharmacokinetics. In Applied Pharmacology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 17–34. ISBN 978-1-4377-0310-8. [Google Scholar]
- Di, L.; Kerns, E.H. Pharmacokinetics. In Drug-Like Properties; Elsevier: Amsterdam, The Netherlands, 2016; pp. 267–281. ISBN 978-0-12-801076-1. [Google Scholar]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.-H. Mathematical Modeling and Simulation of Drug Release from Microspheres: Implications to Drug Delivery Systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsay, R.-Y. Drug Release from a Spherical Matrix: Theoretical Analysis for a Finite Dissolution Rate Affected by Geometric Shape of Dispersed Drugs. Pharmaceutics 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Farmacopéia Brasileira; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2010.
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Kiparissides, C. Polymerization Reactor Modeling: A Review of Recent Developments and Future Directions. Chem. Eng. Sci. 1996, 51, 1637–1659. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable Polymeric Nanoparticles as Drug Delivery Devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Brooks, B. Suspension Polymerization Processes. Chem. Eng. Technol. 2010, 33, 1737–1744. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Palazzo, A.; Hennink, W.E.; Kok, R.J. Effect of Particle Size on Drug Loading and Release Kinetics of Gefitinib-Loaded PLGA Microspheres. Mol. Pharm. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- Paiva, T.; Melo, P.; Pinto, J.C. Comparative Analysis of Sunscreen Nanoencapsulation Processes. Macromol. Symp. 2016, 368, 60–69. [Google Scholar] [CrossRef]
- Thavanesan, T.; Herbert, C.; Plamper, F.A. Insight in the Phase Separation Peculiarities of Poly(Dialkylaminoethyl Methacrylate)s. Langmuir 2014, 30, 5609–5619. [Google Scholar] [CrossRef] [PubMed]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-Responsive Polymeric Materials: Synthesis, Self-Assembly, and Functional Applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; Bauri, K.; Pal, S.; Goswami, A.; Madras, G.; De, P. Synthesis, Characterization and Thermal Degradation of Dual Temperature- and PH-Sensitive RAFT-Made Copolymers of N,N-(Dimethylamino)Ethyl Methacrylate and Methyl Methacrylate. Polym. Int. 2013, 62, 463–473. [Google Scholar] [CrossRef]
- Jia, J.; Wang, C.; Chen, K.; Yin, Y. Drug Release of Yolk/Shell Microcapsule Controlled by PH-Responsive Yolk Swelling. Chem. Eng. J. 2017, 327, 953–961. [Google Scholar] [CrossRef]
- Song, Z.; Wang, K.; Gao, C.; Wang, S.; Zhang, W. A New Thermo-, PH-, and CO2-Responsive Homopolymer of Poly[N-[2-(Diethylamino)Ethyl]Acrylamide]: Is the Diethylamino Group Underestimated? Macromolecules 2016, 49, 162–171. [Google Scholar] [CrossRef]
- Permanadewi, I.; Kumoro, A.C.; Wardhani, D.H.; Aryanti, N. Modelling of Controlled Drug Release in Gastrointestinal Tract Simulation. J. Phys. Conf. Ser. 2019, 1295, 012063. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Zhao, K.; Yu, X.; Yao, B.; Li, X.; Zha, X.; Zhang, B.; Tan, Q.; Yang, Z.; et al. Self-Expanding PMMA Composite Bone Cement with Sustained Release of Gentamicin Sulfate and Alendronate Using Water Absorption Pathways. Mater. Des. 2022, 222, 111081. [Google Scholar] [CrossRef]
- Valarini Junior, O.; Reitz Cardoso, F.A.; Machado Giufrida, W.; de Souza, M.F.; Cardozo-Filho, L. Production and Computational Fluid Dynamics-Based Modeling of PMMA Nanoparticles Impregnated with Ivermectin by a Supercritical Antisolvent Process. J. CO2 Util. 2020, 35, 47–58. [Google Scholar] [CrossRef]
- Colpo, J.C.; Pigatto, C.; Brizuela, N.; Aragón, J.; dos Santos, L.A.L. Antibiotic and Anesthetic Drug Release from Double-Setting α-TCP Cements. J. Mater. Sci. 2018, 53, 7112–7124. [Google Scholar] [CrossRef]
- Flores, F.P.; Kong, F. In Vitro Release Kinetics of Microencapsulated Materials and the Effect of the Food Matrix. Annu. Rev. Food Sci. Technol. 2017, 8, 237–259. [Google Scholar] [CrossRef]
- Federico, M.P.; Sakata, R.A.P.; Pinto, P.F.C.; Furtado, G.H.C. Noções Sobre Parâmetros Farmacocinéticos/Farmacodinâmicos e Sua Utilização Na Prática Médica 2017. Rev. Soc. Bras. Clínica Médica 2017, 15, 201–205. [Google Scholar]
- Durrer, C.; Irache, J.M.; Puisieux, F.; Duchêne, D.; Ponchel, G. Mucoadhesion of Latexes. I. Analytical Methods and Kinetic Studies. Pharm. Res. 1994, 11, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The Mechanism of Uptake of Biodegradable Microparticles in Caco-2 Cells Is Size Dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Delie, F.; Blanco-Príeto, M. Polymeric Particulates to Improve Oral Bioavailability of Peptide Drugs. Molecules 2005, 10, 65–80. [Google Scholar] [CrossRef] [PubMed]




| Sample | Average Particle Size (Total) | SPAN (Total) * | Average Particle Size (<106 μm) | SPAN (<106 μm) |
|---|---|---|---|---|
| PMMA-Nano | 170 ± 1.3 nm | 0.90 | - | - |
| PMMA-Micro | 89.9 ± 0.2 μm | 1.5 | 69.4 ± 0.7 μm | 0.78 |
| PMMA-co-DEAEMA | 27 ± 1 μm | 7.2 | 28.7 ± 3 μm | 2.3 |
| PMMA-co-DMAEMA | 173 ± 11 μm | 2.6 | 63.7 ± 0.6 μm | 1.3 |
| Sample | Concentration (mg/mL) | Entrapment Efficiency (%) |
|---|---|---|
| PMMA-Nano | 0.60 ± 0.02 | 100 ± 4 |
| PMMA-Micro | 0.80 ± 0.10 | 100 ± 9 |
| PMMA-co-DEAEMA | 0.58 ± 0.03 | 96 ± 6 |
| PMMA-co-DMAEMA | 0.60 ± 0.01 | 99 ± 2 |
| Model | Sample | R2 | MSE |
|---|---|---|---|
| Zero-order | PMMA-Micro | 0.68 | 18 |
| PMMA-co-DEAEMA | −1.27 | 1941 | |
| PMMA-co-DMAEMA | −0.65 | 1250 | |
| First-order | PMMA-Micro | 0.71 | 16 |
| PMMA-co-DEAEMA | −0.05 | 893 | |
| PMMA-co-DMAEMA | 0.20 | 603 | |
| Higuchi | PMMA-Micro | 0.78 | 12 |
| PMMA-co-DEAEMA | 0.09 | 776 | |
| PMMA-co-DMAEMA | 0.42 | 435 | |
| Korsmeyer-Peppas | PMMA-Micro | 0.79 | 1 |
| PMMA-co-DEAEMA | 0.99 | 9 | |
| PMMA-co-DMAEMA | 0.86 | 128 | |
| Hixson-Crowell | PMMA-Micro | 0.70 | 17 |
| PMMA-co-DEAEMA | −0.28 | 1090 | |
| PMMA-co-DMAEMA | 0.01 | 748 | |
| Hopfenberg | PMMA-Micro | 0.70 | 20 |
| PMMA-co-DEAEMA | −0.28 | 1308 | |
| PMMA-co-DMAEMA | 0.05 | 866 | |
| Peppas-Sahlin | PMMA-Micro | 0.71 | 24 |
| PMMA-co-DEAEMA | 0.83 | 220 | |
| PMMA-co-DMAEMA | 0.96 | 40 |
| Model | Parameter | PMMA-co-DEAEMA | PMMA-co- DMAEMA | PMMAMicroo |
|---|---|---|---|---|
| Korsmeyer-Peppas | Kkp | 54 | 23 | 0.77 |
| n | 0.09 | 0.25 | 0.61 | |
| Peppas-Sahlin | kd | 23 | 19 | 2.18 |
| kr | −1.5 | −1.2 | −0.06 | |
| m | 0.45 | 0.45 | 0.45 |
| Parameters 1 | PZQ Free | PMMANano | PMMAMicro | PMMA-co-DEAEMA | PMMA-co-DMAEMA |
|---|---|---|---|---|---|
| Cmax (ng/mL) | 432 ± 98 | 286 ± 14 | 42 ± 19 | 1007 ± 83 | 134 ± 59 |
| AUClast 2 (h*ng/mL) | 1012 ± 424 | 1012 ± 166 | 2 ± 9 | 1063 ± 390 | 320 ± 94 |
| AUCINF_obs 3 (h*ng/mL) | 1302 ± 525 | 5341 ± 1386 | - | 1677 ± 384 | - |
| Ke (1/h) 3 | 0.25 ± 0.08 | 0.03 ± 0.01 | - | 0.34 ± 0.08 | - |
| Tmax (h) | 0.25 | 0.41 ± 0.08 | 8 | 0.33 ± 0.08 | 17.00 ± 7.00 |
| T1/2β (h) 3 | 3.64 ± 1.46 | 6.20 ± 1.96 | - | 2.24 ± 0.46 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, E.D.; da Silva Dutra, L.; Paiva, T.F.; de Almeida Carvalho, L.L.; Rocha, H.V.A.; Pinto, J.C. In Vitro Release and In Vivo Pharmacokinetics of Praziquantel Loaded in Different Polymer Particles. Materials 2023, 16, 3382. https://doi.org/10.3390/ma16093382
Pereira ED, da Silva Dutra L, Paiva TF, de Almeida Carvalho LL, Rocha HVA, Pinto JC. In Vitro Release and In Vivo Pharmacokinetics of Praziquantel Loaded in Different Polymer Particles. Materials. 2023; 16(9):3382. https://doi.org/10.3390/ma16093382
Chicago/Turabian StylePereira, Emiliane Daher, Luciana da Silva Dutra, Thamiris Franckini Paiva, Larissa Leite de Almeida Carvalho, Helvécio Vinícius Antunes Rocha, and José Carlos Pinto. 2023. "In Vitro Release and In Vivo Pharmacokinetics of Praziquantel Loaded in Different Polymer Particles" Materials 16, no. 9: 3382. https://doi.org/10.3390/ma16093382