Application of Silsesquioxanes in the Preparation of Polyolefin-Based Materials
Abstract
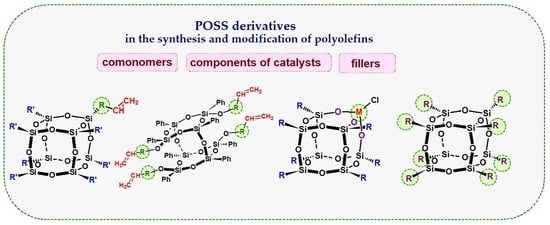
:1. Introduction
2. Silsesquioxane Moiety in Precatalysts for Olefin Polymerization
2.1. POSS as a Carrier Modifier and Homogeneous Support
2.2. POSS as a Ligand in Transition Metal Complexes
3. Copolymerization of Ethylene with Alkenylsilsesquioxanes over Organometallic Catalysts
3.1. Ethylene Copolymerization with Mono-Alkenylsilsesquioxanes
3.2. Ethylene Copolymerization with Di-, Tri- and Tetra-Alkenylsilsesquioxanes
4. Silsesquioxane Derivates as Fillers in Composites Based on Polyolefins
4.1. Polyolefin/POSS Nanocomposites
4.2. Siloxane-Silsesquioxane Resins as Fillers of Polyolefin Nanocomposites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts 2022. Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 20 February 2023).
- Nwabunma, D.; Kyu, T. Polyolefin Composites; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-19902-2. [Google Scholar]
- Kickelbick, G. Introduction to Hybrid Materials. In Hybrid Materials. Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–48. ISBN 978-3-527-61049-5. [Google Scholar]
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; ISBN 978-90-481-3787-9. [Google Scholar]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral Oligomeric Silsesquioxane-Based Hybrid Materials and Their Applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Xu, H.; Kuo, S.-W.; Lee, J.-S.; Chang, F.-C. Preparations, Thermal Properties, and Tg Increase Mechanism of Inorganic/Organic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2002, 35, 8788–8793. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K. Coordination Polymerization of Alkenylsilsesquioxanes and Their Copolymerization with Olefins or Styrene. Polimery-W 2015, 60, 289–297. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K. Silsesquioxanes and Their Application in the Synthesis of Polymeric Materials. Polimery-W 2015, 60, 219–231. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Feher, F.J.; Tajima, T.L. Synthesis of a Molybdenum-Containing Silsesquioxane Which Rapidly Catalyzes the Metathesis of Olefins. J. Am. Chem. Soc. 1994, 116, 2145–2146. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Czaban, J.; Krishnan, G.R.; Malińska, M.; Woźniak, K.; Siddique, H.; Peeva, L.G.; Livingston, A.G.; Grela, K. Batchwise and Continuous Nanofiltration of POSS-Tagged Grubbs–Hoveyda-Type Olefin Metathesis Catalysts. ChemSusChem 2013, 6, 182–192. [Google Scholar] [CrossRef]
- Abbenhuis, H.C.L.; Krijnen, S.; van Santen, R.A. Modelling the Active Sites of Heterogeneous Titanium Epoxidation Catalysts Using Titanium Silasequioxanes: Insight into Specific Factors That Determine Leaching in Liquid-Phase Processes. Chem. Commun. 1997, 331–332. [Google Scholar] [CrossRef] [Green Version]
- Crocker, M.; Herold, R.H.M.; Crocker, M.; Orpen, A.G. Synthesis and Structural Characterisation of Tripodal Titanium Silsesquioxane Complexes: A New Class of Highly Active Catalysts for Liquid Phase Alkene Epoxidation. Chem. Commun. 1997, 2411–2412. [Google Scholar] [CrossRef]
- Fujiwara, M.; Wessel, H.; Hyung-Suh, P.; Roesky, H.W. Formation of Titanium Tert-Butylperoxo Intermediate from Cubic Silicon–Titanium Complex with Tert-Butyl Hydroperoxide and Its Reactivity for Olefin Epoxidation. Tetrahedron 2002, 58, 239–243. [Google Scholar] [CrossRef]
- Leng, Y.; Zhao, J.; Jiang, P.; Wang, J. Amphiphilic Porous Polyhedral Oligomeric Silsesquioxanes (POSS) Incorporated Polyoxometalate-Paired Polymeric Hybrids: Interfacial Catalysts for Epoxidation Reactions. RSC Adv. 2015, 5, 17709–17715. [Google Scholar] [CrossRef]
- Vieira, E.G.; Dal-Bó, A.G.; Frizon, T.E.A.; Dias Filho, N.L. Synthesis of Two New Mo(II) Organometallic Catalysts Immobilized on POSS for Application in Olefin Oxidation Reactions. J. Organomet. Chem. 2017, 834, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Zhao, J.; Leng, Y.; Jiang, P.; Zhang, C. Novel Porous and Hydrophobic POSS-Ionic Liquid Polymeric Hybrid as Highly Efficient Solid Acid Catalyst for Synthesis of Oleate. Catal. Commun. 2016, 83, 27–30. [Google Scholar] [CrossRef]
- Leng, Y.; Zhao, J.; Jiang, P.; Lu, D. POSS-Derived Solid Acid Catalysts with Excellent Hydrophobicity for Highly Efficient Transformations of Glycerol. Catal. Sci. Technol. 2016, 6, 875–881. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F.; Atai, M. Pd on Magnetic Hybrid of Halloysite and POSS-Containing Copolymer: An Efficient Catalyst for Dye Reduction. Appl. Organomet. Chem. 2020, 34, e6006. [Google Scholar] [CrossRef]
- Akbari, A.; Naderahmadian, A.; Eftekhari-Sis, B. Silver and Copper Nanoparticles Stabilized on Ionic Liquids-Functionalized Polyhedral Oligomeric Silsesquioxane (POSS): Highly Active and Recyclable Hybrid Catalysts. Polyhedron 2019, 171, 228–236. [Google Scholar] [CrossRef]
- Xia, S.; Yang, Y.; Lü, C. Quaternized POSS Modified RGO-Supported Pd Nanoparticles as a Highly Efficient Catalyst for Reduction and Suzuki Coupling Reactions. New J. Chem. 2019, 43, 18601–18610. [Google Scholar] [CrossRef]
- Ismagilov, I.Z.; Matus, E.V.; Kuznetsov, V.V.; Yashnik, S.A.; Kerzhentsev, M.A.; Gerritsen, G.; Abbenhuis, H.C.L.; Ismagilov, Z.R. Application of POSS Nanotechnology for Preparation of Efficient Ni Catalysts for Hydrogen Production. Eurasian Chem.-Technol. J. 2017, 19, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, D.; Galland, G.B.; dos Santos, J.H.Z.; Williams, R.J.J.; Fasce, D.P.; Dell’Erba, I.E.; Quijada, R.; Perez, M. Metallocene Supported on a Polyhedral Oligomeric Silsesquioxane-Modified Silica with High Catalytic Activity for Ethylene Polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5465–5476. [Google Scholar] [CrossRef]
- García-Orozco, I.; Velilla, T.; Galland, G.B.; dos Santos, J.H.Z.; Williams, R.J.J.; Quijada, R. Metallocene supported on a polyhedral oligomeric silsesquioxane-modified silica: Structural characterization and catalytic activity for ethylene polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5938–5944. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Zhang, J.; Mu, J.; Gong, D.; Wang, X. Immobilization of Isolated FI Catalyst on Polyhedral Oligomeric Silsesquioxane-Functionalized Silica for the Synthesis of Weakly Entangled Polyethylene. Chem. Commun. 2016, 52, 11092–11095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, L.; Yue, Z.; Yang, H.; Chen, T.; Li, W. Influence of the Fragmentation of POSS-Modified Heterogeneous Catalyst on the Formation of Chain Entanglements. Ind. Eng. Chem. Res. 2018, 57, 9400–9406. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Ribeiro, M.R.; Cramail, H.; Lourenço, J.P.; Lorenzo, V.; Pérez, E.; Cerrada, M.L. Extraordinary Mechanical Performance in Disentangled UHMWPE Films Processed by Compression Molding. J. Mech. Behav. Biomed. Mater. 2019, 90, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, P.; Yue, Z.; Li, W.; Dong, C.; Jiang, B.; Wang, J.; Yang, Y. Entanglement Formation Mechanism in the POSS Modified Heterogeneous Ziegler–Natta Catalysts. Macromolecules 2019, 52, 7593–7602. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, M.; Chen, Y.; Li, W.; Zhou, Q.; Dai, J.; Ye, S.; Jiang, B.; Wang, J.; Yang, Y. Suppressing the Entanglements of Ultrahigh-Molecular-Weight Polyethylene via Controlling the Adhesion Effect in a POSS-Modified Support. Ind. Eng. Chem. Res. 2022, 61, 6367–6374. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, N.; Cao, Y.; Li, W.; Dong, C. Reduced Entanglement Density of Ultrahigh-Molecular-Weight Polyethylene Favored by the Isolated Immobilization on the MgCl2 (110) Plane. Ind. Eng. Chem. Res. 2020, 59, 3351–3358. [Google Scholar] [CrossRef]
- Li, W.; Hui, L.; Xue, B.; Dong, C.; Chen, Y.; Hou, L.; Jiang, B.; Wang, J.; Yang, Y. Facile High-Temperature Synthesis of Weakly Entangled Polyethylene Using a Highly Activated Ziegler-Natta Catalyst. J. Catal. 2018, 360, 145–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Z. Homogeneous Polyhedral Oligomeric Silsesquioxane (POSS)-Supported Pd–Diimine Complex and Synthesis of Polyethylenes End-Tethered with a POSS Nanoparticle via Ethylene “Living” Polymerization. Chem. Commun. 2008, 1178–1180. [Google Scholar] [CrossRef]
- Severn, J.R.; Duchateau, R.; van Santen, R.A.; Ellis, D.D.; Spek, A.L.; Yap, G.P.A. Silsesquioxane-Bonded Zirconocene Complexes; Soluble Models for Silica-Tethered Olefin Polymerization Catalysts. Dalton Trans. 2003, 2293–2302. [Google Scholar] [CrossRef] [Green Version]
- Severn, J.R.; Duchateau, R.; van Santen, R.A.; Ellis, D.D.; Spek, A.L. Homogeneous Models for Chemically Tethered Silica-Supported Olefin Polymerization Catalysts. Organometallics 2002, 21, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, R.W.J.M.; van Santen, R.A.; Abbenhuis, H.C.L. The Dynamic Status Quo of Polyhedral Silsesquioxane Coordination Chemistry. Eur. J. Inorg. Chem. 2004, 2004, 675–683. [Google Scholar] [CrossRef]
- Pochwała, M.; Białek, M. Zastosowanie Kompleksów Metali Przejściowych z Ligandami POSS w Polimeryzacji Olefin. In Na Pograniczu Chemii i Biologii; Koroniak, H., Braciszewski, J., Eds.; Wydawnictwo Naukowe UAM: Poznan, Poland, 2014; Volume XXXIII, pp. 13–22. ISBN 978-83-232-2803-5. [Google Scholar]
- Duchateau, R.; Cremer, U.; Harmsen, R.J.; Mohamud, S.I.; Abbenhuis, H.C.L.; van Santen, R.A.; Meetsma, A.; Thiele, S.K.-H.; van Tol, M.F.H.; Kranenburg, M. Half-Sandwich Group 4 Metal Siloxy and Silsesquioxane Complexes: Soluble Model Systems for Silica-Grafted Olefin Polymerization Catalysts. Organometallics 1999, 18, 5447–5459. [Google Scholar] [CrossRef]
- Duchateau, R.; Dijkstra, T.W.; van Santen, R.A.; Yap, G.P.A. Silsesquioxane Models for Silica Surface Silanol Sites with Adjacent Siloxide Functionalites and Olefin Polymerization Catalysts Thereof. Chem. Eur. J. 2004, 10, 3979–3990. [Google Scholar] [CrossRef]
- Duchateau, R.; Abbenhuis, H.C.L.; van Santen, R.A.; Thiele, S.K.-H.; van Tol, M.F.H. Half-Sandwich Titanium Complexes Stabilized by a Novel Silsesquioxane Ligand: Soluble Model Systems for Silica-Grafted Olefin Polymerization Catalysts. Organometallics 1998, 17, 5222–5224. [Google Scholar] [CrossRef]
- Varga, V.; Pinkas, J.; Gyepes, R.; Štěpnička, P.; Horáček, M.; Bastl, Z.; Mach, K. Synthesis of Zirconocene Silsesquioxane Complexes and Their Ethene Polymerization Activity in Systems with Methylaluminoxane. Collect. Czech. Chem. Commun. 2010, 75, 105–119. [Google Scholar] [CrossRef]
- Mehta, A.; Tembe, G.; Białek, M.; Parikh, P.; Mehta, G. Synthesis, Characterization and Ethylene Polymerization by Metallasilsesquioxane. Polym. Adv. Technol. 2013, 24, 441–445. [Google Scholar] [CrossRef]
- Mehta, A.M.; Tembe, G.L.; Parikh, P.A.; Mehta, G.N. Catalytic Ethylene Polymerization by the Titanium-Polyhedral Oligomeric Silsesquioxane-Et3Al2Cl3 System. React. Kinet. Mech. Catal. 2011, 104, 369–375. [Google Scholar] [CrossRef]
- Duchateau, R.; Abbenhuis, H.C.L.; van Santen, R.A.; Meetsma, A.; Thiele, S.K.-H.; van Tol, M.F.H. Ethylene Polymerization with Dimeric Zirconium and Hafnium Silsesquioxane Complexes. Organometallics 1998, 17, 5663–5673. [Google Scholar] [CrossRef]
- Liu, J.-C. A Bimetallic Siloxane Cage Model Catalyst. Synthesis, Characterization and Polymerization Behaviour of [(c-C6H11)7(Si7O12 )MgTiCl3]n (n = 1,2). Chem. Commun. 1996, 1109–1110. [Google Scholar] [CrossRef]
- Pochwała, M.; Białek, M.; Franczyk, A.; Marciniec, B.; Czaja, K. Synthesis and Catalytic Performance in Ethylene and 1-Octene Polymerization of Chlorotitanium(IV) Silsesquioxane Complexes. Effect of Increasing Ligand Denticity and Type of Nonreactive Organic Substituents. Eur. Polym. J. 2016, 79, 121–131. [Google Scholar] [CrossRef]
- Pochwała, M.; Białek, M.; Franczyk, A.; Czaja, K.; Marciniec, B. Synthesis and Catalytic Behavior in Olefin Polymerization of Bimetallic Titanium(IV) Silsesquioxane Complex and Its Polymeric Counterpart. Polimery-W 2016, 61, 591–599. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A. Silasesquioxanes as Ligands in Inorganic and Organometallic Chemistry. Polyhedron 1995, 14, 3239–3253. [Google Scholar] [CrossRef]
- Feher, F.J.; Walzer, J.F.; Blanski, R.L. Olefin Polymerization by Vanadium-Containing Polyhedral Oligometallasilsesquioxanes. J. Am. Chem. Soc. 1991, 113, 3618–3619. [Google Scholar] [CrossRef]
- Feher, F.J.; Blanski, R.L. Olefin Polymerization by Vanadium-Containing Silsesquioxanes: Synthesis of a Dialkyl-Oxo-Vanadium(V) Complex That Initiates Ethylene Polymerization. J. Am. Chem. Soc. 1992, 114, 5886–5887. [Google Scholar] [CrossRef]
- Ohde, C.; Limberg, C.; Schmidt, D.; Enders, M.; Demeshko, S.; Knispel, C. Progress in the Compilation of an Oxovanada-Silsesquioxane Portfolio and Catalytic Activity of Organometallic Representatives in Ethylene Polymerisation. Z. Anorg. Allg. Chem. 2010, 636, 2315–2322. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Pochwała, M.; Franczyk, A.; Czaja, K.; Marciniec, B. Synthesis and Catalytic Properties for Olefin Polymerization of New Vanadium Complexes Containing Silsesquioxane Ligands with Different Denticity. Polym. Int. 2017, 66, 960–967. [Google Scholar] [CrossRef]
- Feher, F.J.; Blanski, R.L. Polyhedral Oligometallasilasesquioxanes as Models for Silica-Supported Catalysts: Chromium Attached to Two Vicinal Siloxy Groups. J. Chem. Soc., Chem. Commun. 1990, 1614–1616. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, S.; Terano, M. Silsesquioxane-Supported Chromium Catalyst for Insight into Phillips-Type Ethylene Polymerization. Macromol. React. Eng. 2018, 12, 1800049. [Google Scholar] [CrossRef]
- Baba, R.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T. The Influence of Functional Groups on the Ethylene Polymerization Performance of Silsesquioxane-Supported Phillips-Type Catalysts. Dalton Trans. 2017, 46, 12158–12166. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-W.; Chang, F.-C. POSS Related Polymer Nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Liu, N.; Zheng, S. Organic–Inorganic Poly(N-Vinylpyrrolidone) Copolymers with Double-Decker Silsesquioxane in the Main Chains: Synthesis, Glass Transition, and Self-Assembly Behavior. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2949–2961. [Google Scholar] [CrossRef]
- Kozuma, T.; Mihata, A.; Kaneko, Y. Preparation of Soluble POSS-Linking Polyamide and Its Application in Antifogging Films. Materials 2021, 14, 3178. [Google Scholar] [CrossRef]
- Neyertz, S.; Salimi, S.; Radmanesh, F.; Benes, N.E.; Brown, D. High-Temperature Molecular Screening of Hybrid PolyOAPS-Imide Networks Based on Octa(Aminophenyl)Silsesquioxane for Increased Thermomechanical Resistance. Phys. Chem. Chem. Phys. 2021, 23, 11438–11454. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; Wang, L.; Zheng, S. Organic-Inorganic Polybenzoxazine Copolymers with Double Decker Silsesquioxanes in the Main Chains: Synthesis and Thermally Activated Ring-Opening Polymerization Behavior. Polymer 2017, 109, 254–265. [Google Scholar] [CrossRef]
- Zhao, B.; Mei, H.; Zheng, S. Polyethylene Telechelics with POSS Termini: Synthesis, Morphologies and Shape Memory Properties. Polym. Chem. 2020, 11, 5819–5832. [Google Scholar] [CrossRef]
- Chae, C.-G.; Yu, Y.-G.; Seo, H.-B.; Kim, M.-J.; Kishore, M.Y.L.N.; Lee, J.-S. Molecular and Kinetic Design for the Expanded Control of Molecular Weights in the Ring-Opening Metathesis Polymerization of Norbornene-Substituted Polyhedral Oligomeric Silsesquioxanes. Polym. Chem. 2018, 9, 5179–5189. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Zheng, S. Synthesis of POSS-Terminated Polycyclooctadiene Telechelics via Ring-Opening Metathesis Polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 223–233. [Google Scholar] [CrossRef]
- Ata, S.; Banerjee, S.L.; Singha, N.K. Self-Assembly Behavior of POSS Based ABA Type Amphiphilic Tri-Block Copolymer Prepared via ATRP. Eur. Polym. J. 2019, 118, 10–16. [Google Scholar] [CrossRef]
- Ata, S.; Banerjee, S.L.; Singha, N.K. Polymer Nano-Hybrid Material Based on Graphene Oxide/POSS via Surface Initiated Atom Transfer Radical Polymerization (SI-ATRP): Its Application in Specialty Hydrogel System. Polymer 2016, 103, 46–56. [Google Scholar] [CrossRef]
- Ullah, A.; Ahmad, S.; Maric, M.; Shah, S.M.; Hussain, H. Low Temperature ATRP of POSS-MA and Its Amphiphilic Pentablock Copolymers. J. Polym. Sci. 2022, 60, 2488–2499. [Google Scholar] [CrossRef]
- Tsuchida, A.; Bolln, C.; Sernetz, F.G.; Frey, H.; Mülhaupt, R. Ethene and Propene Copolymers Containing Silsesquioxane Side Groups. Macromolecules 1997, 30, 2818–2824. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Jung, M.-S.; Shin, Y.-J.; Yoon, K.-B.; Lee, D.-H. Preparation and Properties of Ethylene/POSS Copolymer with Rac-Et(Ind)2ZrCl2 Catalyst. J. Appl. Polym. Sci. 2009, 111, 2697–2702. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Shin, Y.-J.; Yoon, K.-B.; Lee, D.-H. Preparation and Properties of Propylene/POSS Copolymer with Rac-Et(Ind)2ZrCl2 Catalyst. Eur. Polym. J. 2009, 45, 40–46. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Dudziec, B.; Marciniec, B. Copolymers of Ethylene with Monoalkenyl- and Monoalkenyl(Siloxy)Silsesquioxane (POSS) Comonomers—Synthesis and Characterization. Eur. Polym. J. 2017, 90, 368–382. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Mituła, K.; Dudziec, B. Multi-Alkenylsilsesquioxanes as Comonomers and Active Species Modifiers of Metallocene Catalyst in Copolymerization with Ethylene. Polymers 2018, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Groch, P.; Dziubek, K.; Czaja, K.; Białek, M.; Mituła, K.; Dudziec, B.; Marciniec, B. Synthesis and Structural Characterization of Ethylene Copolymers Containing Double-Decker Silsesquioxane as Pendant Groups and Cross-Linkage Sites by Coordinative Copolymerization. Eur. Polym. J. 2018, 100, 187–199. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Białek, M.; Man, D. Tri-Alkenyl Polyhedral Oligomeric Silsesquioxanes as Comonomers and Active Center Modifiers in Ethylene Copolymerization Catalyzed by Bis(Phenoxy-Imine) Ti, Zr, V and V Salen-Type Complexes. Appl. Catal. A Gen. 2018, 567, 122–131. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Białek, M.; Adamczyk-Tomiak, K.; Rabiej, S.; Dudziec, B. Ethylene/POSS Copolymerization Behavior of Postmetallocene Catalysts and Copolymer Characteristics. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3918–3934. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Grzymek, M. Investigation of Thermal Stability of Ethylene Copolymers with POSS—Study under Static and Dynamic Conditions. Polym. Degrad. Stab. 2018, 156, 218–227. [Google Scholar] [CrossRef]
- Groch, P.; Czaja, K.; Sacher-Majewska, B. Thermal Stability of Ethylene Copolymers with Multi-Alkenylsilsesquioxane Comonomers Synthesized by Organometallic Catalyst—Effect of Copolymer Structure. Polym. Degrad. Stab. 2020, 172, 109075. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Sacher-Majewska, B. Thermal Behavior of Ethylene Copolymers with Di- and Tri-Alkenylsilsesquioxane Comonomers Synthesized by Post-Metallocene Catalysts. J. Therm. Anal. Calorim. 2020, 142, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Groch, P.; Czaja, K.; Szeluga, U.; Rabiej, S.; Bączek, M. The Effect of Macromolecular Architecture of Ethylene Copolymers with Multi-Alkenylsilsesquioxane on Morphological, Rheological and Dynamic Mechanical Behavior. Polymer 2021, 212, 123172. [Google Scholar] [CrossRef]
- Caseri, W. Nanocomposites of Polymers and Inorganic Particles. In Hybrid Materials: Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 49–86. [Google Scholar] [CrossRef]
- Dougnac, V.N.; Alamillo, R.; Peoples, B.C.; Quijada, R. Effect of Particle Diameter on the Permeability of Polypropylene/SiO2 Nanocomposites. Polymer 2010, 51, 2918–2926. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Chen, G.; Wu, L. Preparation and Characterization of Polyester/Silica Nanocomposite Resins. Prog. Org. Coat. 2005, 54, 120–126. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990; ISBN 978-0-08-057103-4. [Google Scholar]
- Imai, Y. Inorganic Nano-Fillers for Polymers. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomaterials 2012, 2012, 132424. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Branda, F. The Sol-Gel Route to Nanocomposites. In Advances in Nanocomposites-Synthesis, Characterization and Industrial Applications; Reddy, B., Ed.; IntechOpen: London, UK, 2011; Volume 14, pp. 323–340. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Deng, T.; Zhang, G. Preparation and Size Control of Highly Monodisperse Vinyl Functionalized Silica Spheres. Appl. Surf. Sci. 2012, 258, 1910–1914. [Google Scholar] [CrossRef]
- Vansant, E.F.; Van Der Voort, P.; Vrancken, K.C. Characterization and Chemical Modification of the Silica Surface; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 0-08-052895-3. [Google Scholar]
- Wei, L.; Hu, N.; Zhang, Y. Synthesis of Polymer—Mesoporous Silica Nanocomposites. Materials 2010, 3, 4066–4079. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Park, J.-H.; Oh, S.-G.; Kim, Y.C. Preparation of Highly Monodispersed Hybrid Silica Spheres Using a One-Step Sol−Gel Reaction in Aqueous Solution. Langmuir 2007, 23, 10875–10878. [Google Scholar] [CrossRef] [PubMed]
- Markovic, E.; Constantopolous, K.; Matisons, J.G. Polyhedral Oligomeric Silsesquioxanes: From Early and Strategic Development through to Materials Application. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2011; pp. 1–46. ISBN 978-90-481-3786-2. [Google Scholar]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral Oligomeric Silsesquioxanes (POSS)-Containing Nanohybrid Polymers. In Supramolecular Polymers Polymeric Betains Oligomers. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 201, pp. 225–296. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS Polymers: Physical Properties and Biomaterials Applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Njuguna, J.; Ansari, F.; Sachse, S.; Rodriguez, V.M.; Siqqique, S.; Zhu, H. Nanomaterials, Nanofillers, and Nanocomposites: Types and Properties. In Health and Environmental Safety of Nanomaterials, 2nd ed.; Njuguna, J., Pielichowski, K., Zhu, H., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–37. ISBN 978-0-12-820505-1. [Google Scholar]
- Gnanasekaran, D.; Madhavan, K.; Reddy, B.S.R. Developments of Polyhedral Oligomeric Silsesquioxanes (POSS), POSS Nanocomposites and Their Applications: A Review. J. Sci Ind. Res. 2009, 68, 437–464. [Google Scholar]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [Green Version]
- Diao, S.; Mao, L.; Zhang, L.; Wang, Y. POSS/Polyurethane Hybrids and Nanocomposites: A Review on Preparation, Structure and Performance. Elastomers Compos. 2015, 50, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Kausar, A. State-of-the-Art Overview on Polymer/POSS Nanocomposite. Polym. Plast. Technol. Eng. 2017, 56, 1401–1420. [Google Scholar] [CrossRef]
- Blanco, I. Decomposition and Ageing of Hybrid Materials with POSS. In Polymer/POSS Nanocomposites and Hybrid Materials: Preparation, Properties, Applications; Kalia, S., Pielichowski, K., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 415–462. ISBN 978-3-030-02327-0. [Google Scholar]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, A.; Taghizadeh, M.; Habibzadeh, S.; Jin, Y.; Xiao, H.; Zarrintaj, P.; Saeb, M.R. Polyhedral Oligomeric Silsesquioxane/Epoxy Coatings: A Review. Surf. Innov. 2021, 9, 3–16. [Google Scholar] [CrossRef]
- Ullah, M.S.; Yazici, N.; Wis, A.A.; Abdulmounem, A.; Ozkoc, G.; Kodal, M. A Review on Polyhedral Oligomeric Silsesquioxanes as a New Multipurpose Nanohybrid Additive for Poly(Lactic Acid) and Poly(Lactic Acid) Hybrid Composites. Polym. Compos. 2022, 43, 1252–1281. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Lichtenhan, J.D. Polyhedral Oligomeric Silsesquioxane Induced Thermomechanical Reinforcement in Polymer Films Using Only Parts-per-Million Content. Macromol. Mater. Eng. 2020, 305, 2000354. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Lichtenhan, J.; Reyes-Mayer, A.; Calixto-Rodriguez, M.; Sarmiento-Bustos, E.; Yañez-Lino, M. Parts-per-Million Polyhedral Oligomeric Silsesquioxane Loading Induced Mechanical Reinforcement in Polyethylene Nanocomposites. When Small and Well-Dispersed Yields Big. Polym. Adv. Technol. 2020, 31, 2453–2465. [Google Scholar] [CrossRef]
- Scapini, P.; Figueroa, C.A.; Amorim, C.L.; Machado, G.; Mauler, R.S.; Crespo, J.S.; Oliveira, R.V. Thermal and Morphological Properties of High-Density Polyethylene/Ethylene–Vinyl Acetate Copolymer Composites with Polyhedral Oligomeric Silsesquioxane Nanostructure. Polym. Int. 2010, 59, 175–180. [Google Scholar] [CrossRef]
- Fu, B.X.; Yang, L.; Somani, R.H.; Zong, S.X.; Hsiao, B.S.; Phillips, S.; Blanski, R.; Ruth, P. Crystallization Studies of Isotactic Polypropylene Containing Nanostructured Polyhedral Oligomeric Silsesquioxane Molecules under Quiescent and Shear Conditions. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2727–2739. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene–Polyhedral Oligomeric Silsesquioxanes (POSS) Nanocomposites. Polymer 2005, 46, 7855–7866. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chiou, Y.-D. Crystallization Behavior and Morphological Development of Isotactic Polypropylene Blended with Nanostructured Polyhedral Oligomeric Silsesquioxane Molecules. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2122–2134. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S. Studies on Nonisothermal Crystallization of HDPE/POSS Nanocomposites. Polymer 2004, 45, 4953–4968. [Google Scholar] [CrossRef]
- Pracella, M.; Chionna, D.; Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene-POSS Nanocomposites: Morphology and Crystallization Behaviour. Macromol. Symp. 2006, 234, 59–67. [Google Scholar] [CrossRef]
- Baldi, F.; Bignotti, F.; Fina, A.; Tabuani, D.; Ricco, T. Mechanical Characterization of Polyhedral Oligomeric Silsesquioxane/Polypropylene Blends. J. Appl. Polym. Sci. 2007, 105, 935–943. [Google Scholar] [CrossRef]
- Chen, J.-H.; Yao, B.-X.; Su, W.-B.; Yang, Y.-B. Isothermal Crystallization Behavior of Isotactic Polypropylene Blended with Small Loading of Polyhedral Oligomeric Silsesquioxane. Polymer 2007, 48, 1756–1769. [Google Scholar] [CrossRef]
- Frone, A.N.; Perrin, F.X.; Radovici, C.; Panaitescu, D.M. Influence of Branched or Un-Branched Alkyl Substitutes of POSS on Morphology, Thermal and Mechanical Properties of Polyethylene. Compos. Part B-Eng. 2013, 50, 98–106. [Google Scholar] [CrossRef]
- Perrin, F.X.; Panaitescu, D.M.; Frone, A.N.; Radovici, C.; Nicolae, C. The Influence of Alkyl Substituents of POSS in Polyethylene Nanocomposites. Polymer 2013, 54, 2347–2354. [Google Scholar] [CrossRef]
- Bouza, R.; Barral, L.; Díez, F.J.; López, J.; Montero, B.; Rico, M.; Ramírez, C. Study of Thermal and Morphological Properties of a Hybrid System, IPP/POSS. Effect of Flame Retardance. Compos. Part B-Eng. 2014, 58, 566–572. [Google Scholar] [CrossRef]
- Heeley, E.L.; Hughes, D.J.; El Aziz, Y.; Taylor, P.G.; Bassindale, A.R. Morphology and Crystallization Kinetics of Polyethylene/Long Alkyl-Chain Substituted Polyhedral Oligomeric Silsesquioxanes (POSS) Nanocomposite Blends: A SAXS/WAXS Study. Eur. Polym. J. 2014, 51, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Heeley, E.L.; Hughes, D.J.; Taylor, P.G.; Bassindale, A.R. Crystallization and Morphology Development in Polyethylene–Octakis(n-Octadecyldimethylsiloxy)Octasilsesquioxane Nanocomposite Blends. RSC Adv. 2015, 5, 34709–34719. [Google Scholar] [CrossRef] [Green Version]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Dutkiewicz, M.; Marciniec, B. Study of Thermal Properties of Polyethylene and Polypropylene Nanocomposites with Long Alkyl Chain-Substituted POSS Fillers. J. Therm. Anal. Calorim. 2016, 125, 1287–1299. [Google Scholar] [CrossRef] [Green Version]
- Niemczyk, A.; Dziubek, K.; Czaja, K.; Szatanik, R.; Szołyga, M.; Dutkiewicz, M.; Marciniec, B. Polypropylene/Polyhedral Oligomeric Silsesquioxane Nanocomposites—Study of Free Volumes, Crystallinity Degree and Mass Flow Rate. Polimery-W 2016, 61, 610–615. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Czaja, K.; Frączek, D.; Piasecki, R.; Rabiej, S.; Dutkiewicz, M. Study and Evaluation of Dispersion of Polyhedral Oligomeric Silsesquioxane and Silica Filler in Polypropylene Composites. Polym. Compos. 2019, 40, 1354–1364. [Google Scholar] [CrossRef]
- Niemczyk, A.; Adamczyk-Tomiak, K.; Dziubek, K.; Czaja, K.; Rabiej, S.; Szatanik, R.; Dutkiewicz, M. Study of Polyethylene Nanocomposites with Polyhedral Oligomeric Silsesquioxane Nanofillers—From Structural Characteristics to Mechanical Properties and Processability. Polym. Compos. 2019, 40, E350–E364. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yu, F.; Yang, W.; Li, X.; Wang, Z.; Wang, S. C30-45 Long-Chain Alkyls Modified Silsesquioxane: A Promising Additive for Polyethylene. Polym. Compos. 2021, 42, 2205–2216. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene Metal Functionalised POSS Nanocomposites: A Study by Thermogravimetric Analysis. Polym. Degrad. Stab. 2006, 91, 1064–1070. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Camino, G. Metal Functionalized POSS as Fire Retardants in Polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Carniato, F.; Boccaleri, E.; Marchese, L.; Fina, A.; Tabuani, D.; Camino, G. Synthesis and Characterisation of Metal Isobutylsilsesquioxanes and Their Role as Inorganic–Organic Nanoadditives for Enhancing Polymer Thermal Stability. Eur. J. Inorg. Chem. 2007, 2007, 585–591. [Google Scholar] [CrossRef]
- Carniato, F.; Fina, A.; Tabuani, D.; Boccaleri, E. Polypropylene Containing Ti- and Al-Polyhedral Oligomeric Silsesquioxanes: Crystallization Process and Thermal Properties. Nanotechnology 2008, 19, 475701. [Google Scholar] [CrossRef]
- Roy, S.; Lee, B.J.; Kakish, Z.M.; Jana, S.C. Exploiting POSS–Sorbitol Interactions: Issues of Reinforcement of Isotactic Polypropylene Spun Fibers. Macromolecules 2012, 45, 2420–2433. [Google Scholar] [CrossRef]
- Roy, S.; Feng, J.; Scionti, V.; Jana, S.C.; Wesdemiotis, C. Self-Assembled Structure Formation from Interactions between Polyhedral Oligomeric Silsesquioxane and Sorbitol in Preparation of Polymer Compounds. Polymer 2012, 53, 1711–1724. [Google Scholar] [CrossRef]
- Roy, S.; Scionti, V.; Jana, S.C.; Wesdemiotis, C.; Pischera, A.M.; Espe, M.P. Sorbitol–POSS Interactions on Development of Isotactic Polypropylene Composites. Macromolecules 2011, 44, 8064–8079. [Google Scholar] [CrossRef]
- Barczewski, M.; Dobrzyńska-Mizera, M.; Dudziec, B.; Sterzyński, T. Influence of a Sorbitol-based Nucleating Agent Modified with Silsesquioxanes on the Non-isothermal Crystallization of Isotactic Polypropylene. J. Appl. Polym. Sci. 2014, 131, 40131. [Google Scholar] [CrossRef]
- Chen, G.; Feng, B.; Zhu, K.; Zhao, Y.; Yuan, X. Effect of Polyhedral Oligomeric Silsesquioxane and Sorbitol on Properties of Isotactic Polypropylene. Chem. Res. Chin. Univ. 2015, 31, 303–307. [Google Scholar] [CrossRef]
- Mao, H.; Liu, Y.; Liu, W.; Nie, M.; Wang, Q. Investigation of Crystallisation and Interfacial Nature of Polyhedral Oligomeric Silsesquioxane/Polypropylene Composites in the Presence of β-Nucleating Agent. Plast. Rubber Compos. 2017, 46, 322–328. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Carroccio, S.; Curcuruto, G.; Guenzi, M.; Gambarotti, C.; Filippone, G. Multi-Functional Polyhedral Oligomeric Silsesquioxane-Functionalized Carbon Nanotubes for Photo-Oxidative Stable Ultra-High Molecular Weight Polyethylene-Based Nanocomposites. Eur. Polym. J. 2016, 75, 525–537. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Chen, Y.; Yang, B. Preparation of Efficiently Intumescent-Flame-Retarded Polypropylene Composite: Synergistic Effect of Novel Phosphorus-Containing Polyhedral Oligomeric Silsesquioxane. Plast. Rubber Compos. 2021, 50, 464–476. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The Effects of POSS Particles on the Flame Retardancy of Intumescent Polypropylene Composites and the Structure-Property Relationship. Polym. Degrad. Stabil. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Szołyga, M.; Dutkiewicz, M.; Marciniec, B.; Maciejewski, H. Synthesis of Reactive Siloxane-Silsesquioxane Resin. Polimery-W 2013, 58, 766–771. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Polypropylene-based Composites Containing Sorbitol-based Nucleating Agent and Siloxane-silsesquioxane Resin. J. Appl. Polym. Sci. 2016, 133, 43476. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Isotactic Polypropylene Modified with Sorbitol-Based Derivative and Siloxane-Silsesquioxane Resin. Eur. Polym. J. 2016, 85, 62–71. [Google Scholar] [CrossRef]
- Szołyga, M.; Dutkiewicz, M.; Marciniec, B. Polyurethane Composites Based on Silsesquioxane Derivatives of Different Structures. J. Therm. Anal. Calorim. 2018, 132, 1693–1706. [Google Scholar] [CrossRef] [Green Version]
- Niemczyk, A.; Czaja, K.; Dziubek, K.; Szołyga, M.; Rabiej, S.; Szatanik, R. Functionalized Siloxane-silsesquioxane Resins and Polypropylene-based Composites: Morphological, Structural, Thermal, and Mechanical Properties. Polym. Compos. 2019, 40, 3101–3114. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Grzymek, M.; Czaja, K. Accelerated Laboratory Weathering of Polypropylene Composites Filled with Synthetic Silicon-Based Compounds. Polym. Degrad. Stabil. 2019, 161, 30–38. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Czech-Polak, J.; Oliwa, R.; Lenża, J.; Szołyga, M. Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins. Polymers 2018, 10, 1019. [Google Scholar] [CrossRef] [Green Version]
- Niemczyk, A.; Dziubek, K.; Czaja, K.; Szatanik, R. Polyethylene Composites Filled with N-Alkyl-Functionalized Siloxane-Silsesquioxane Resins and Sol-Gel Silicas. Polym. Compos. 2019, 40, 4351–4361. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, M.; Czaja, K. Application of Silsesquioxanes in the Preparation of Polyolefin-Based Materials. Materials 2023, 16, 1876. https://doi.org/10.3390/ma16051876
Białek M, Czaja K. Application of Silsesquioxanes in the Preparation of Polyolefin-Based Materials. Materials. 2023; 16(5):1876. https://doi.org/10.3390/ma16051876
Chicago/Turabian StyleBiałek, Marzena, and Krystyna Czaja. 2023. "Application of Silsesquioxanes in the Preparation of Polyolefin-Based Materials" Materials 16, no. 5: 1876. https://doi.org/10.3390/ma16051876