Investigation of Praseodymium Ions Dopant on 9/65/35 PLZT Ceramics’ Behaviors, Prepared by the Gel-Combustion Route
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
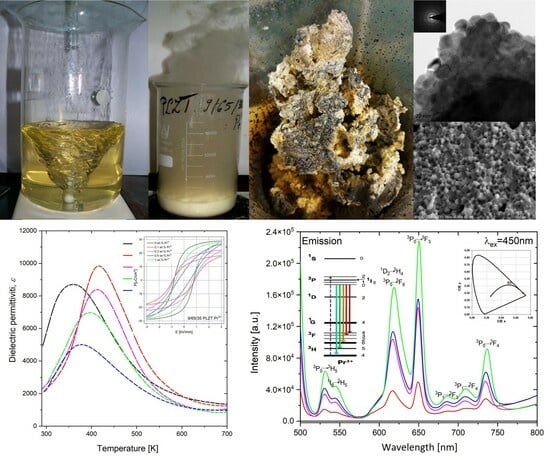
3.1. Thermal Analysis and Electron Microscopy Analysis of Synthesized Powders
3.2. SEM and TEM Analysis of 9/65/35 PLZT:Pr3+ Materials
3.3. X-ray Diffraction Analysis
3.4. 3D Fluorescence Maps, Excitation, and Emission Spectra
3.5. Dielectric and Ferroelectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchino, K. Glory of piezoelectric perovskites. Sci. Technol. Adv. Mater. 2015, 16, 046001. [Google Scholar] [CrossRef] [PubMed]
- Haertling, G.H. Piezoelectric and electrooptic ceramics. In Ceramic Materials for Electronics, Processing, Properties and Applications; Dekker: New York, NY, USA, 2002. [Google Scholar]
- Haertling, G.H. Ferroelectric ceramics: History and technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Płońska, M.; Czekaj, D.; Surowiak, Z. Application of the sol-gel method to the synthesis of ferroelectric nanopowders (Pb1−xLax)(Zr0.65Ti0.35)1−0.25xO3, 0.06 ≤ x ≤ 0.1. Mater. Sci. 2003, 21, 431–437. [Google Scholar]
- Murakami, S.; Morita, M.; Herren, M.; Sakurai, T.; Rau, D. Near-infrared luminescence and spectral anomaly in PLZT ceramics doped with Nd3+, Er3+, Yb3+ and Cr5+ ions at low temperatures. J. Lumin. 2000, 87–89, 694–696. [Google Scholar] [CrossRef]
- Boccaccini, R.A.; Silva, D.D. Industrial Developments in the Field of Optically Transparent Inorganic Materials: A Survey of Recent Patents. Recent Pat. Mater. Sci. 2008, 1, 56–73. [Google Scholar] [CrossRef]
- Qiao, L.; Ye, Q.; Gan, J.L.; Cai, H.W.; Qu, R.H. Optical characteristics of transparent PMNT ceramic and its application at high speed electro-optic switch. Opt. Commun. 2011, 284, 3886–3890. [Google Scholar] [CrossRef]
- Samanta, S.; Muralidhar, M.; Sankaranarayanan, V.; Sethupathi, K.; Ramachandra Rao, M.S.; Murakami, M. Band gap reduction and redshift of lattice vibrational spectra in Nb and Fe co-doped PLZT. J. Mater. Sci. 2017, 52, 13012–13022. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Y.; Tsuboi, T.; Nakai, Y.; Zeng, J.; Li, G. Optical characteristics of Er3+-doped PMN–PT transparent ceramics. Ceram. Int. 2012, 38, 3397–3402. [Google Scholar] [CrossRef]
- Kyômen, T.; Sakamoto, R.; Sakamoto, N.; Kunugi, S.; Itoh, M. Photoluminescence Properties of Pr-Doped (Ca,Sr,Ba)TiO3. Chem. Mater. 2005, 17, 3200–3204. [Google Scholar] [CrossRef]
- Durruthy-Rodríguez, M.D.; Yáñez-Limón, J.M. Photoluminescence in Doped PZT Ferroelectric Ceramic System; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Ramovatar; Coondoo, I.; Satapathy, S.; Kumar, N.; Panwar, N. Dielectric, Piezoelectric Enhancement and Photoluminescent Behavior in Low Temperature Sintered Pr-Modified Ba0.85Ca0.15Zr0.1Ti0.9O3 Ceramics. J. Electron. Mater. 2018, 47, 5870–5878. [Google Scholar] [CrossRef]
- Praveenkumar, B.; Kumar, H.H.; Kharat, D.K.; Murty, B.S. Investigation and characterization of La-doped PZT nanocrystalline ceramic prepared by mechanical activation route. Mater. Chem. Phys. 2008, 112, 31–34. [Google Scholar] [CrossRef]
- Botero, E.R.; Eiras, J.A.; Guo, R.; Bhalla, A.; Garcia, D. Members of Lanthanide Family as Dopants in Relaxor PLZT Ceramics. Itegr. Ferroelectr. 2011, 131, 134–139. [Google Scholar] [CrossRef]
- Okazaki, K.; Masuda, M.; Tashiro, S.; Ishibashi, S. Defect structure and properties of electro-optic PLZT ceramics. Ferroelectrics 1978, 22, 681–682. [Google Scholar] [CrossRef]
- Singh, R.; Goel, T.C.; Chandra, S. RF magnetron sputtered La3+-modified PZT thin films: Perovskite phase stabilization and properties. Mater. Chem. Phys. 2008, 110, 120–127. [Google Scholar] [CrossRef]
- Shannigrahi, S.R.; Choudhary, R.N.P.; Acharya, H.N. Effect of Er doping on structural and dielectric properties of sol-gel prepared PZT ceramics. Mater. Res. Bull. 1999, 34, 1875–1884. [Google Scholar] [CrossRef]
- De Camargo, A.S.S.; Jacinto, C.; Nunes, L.A.O.; Catunda, T.; Garcia, D.; Botero, É.R.; Eiras, J.A. Effect of Nd3+ concentration quenching in highly doped lead lanthanum zirconate titanate transparent ferroelectric ceramics. J. Appl. Phys. 2007, 101, 053111. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, X.; Liu, J.; Feng, Z.; Li, B.; Yang, J.; Li, K.; Jiang, H.; Chen, X.; Xie, J.; et al. Optical properties of Er3+/Yb3+-codoped transparent PLZT ceramic. Physica B 2008, 403, 44–49. [Google Scholar] [CrossRef]
- Ballato, J.; Esmacher, R.; Schwartz, R.; Dejneka, M. Phonon sideband spectroscopy and 1550 nm luminescence from Eu3+ and Er3+-doped ferroelectric PLZT for active electro-optic applications. J. Lumin. 2000, 86, 101–105. [Google Scholar] [CrossRef]
- De Camargo, A.S.S.; de ONunes, L.A.; Santos, I.A.; Garcia, D.; Eiras, J.A. Structural and spectroscopic properties of rare-earth (Nd3+, Er3+,and Yb3+)doped transparent lead lanthanum zirconate titanate ceramics. J. Appl. Phys. 2004, 95, 2135–2140. [Google Scholar] [CrossRef]
- Osińska, K.; Płońska, M.; Marzec, A. Application of the sol-gel method at the fabrication of PLZT:Yb3+ ceramics. Arch. Metall. Mater. 2016, 61, 1441–1446. [Google Scholar] [CrossRef]
- Garcia, D.; Menegazzo, B.A.; Eiras, J.A. Stoichiometric variation and dielectric properties in 9/65/35 PLZT ceramics. Ceramica 1992, 38, 93–96. [Google Scholar]
- Tunaboylu, B.; Harvey, P.; Esener, S.C. Characterization of dielectric and electro-optic properties of PLZT 9/65/35 films on sapphire for electro-optic applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 1105–1112. [Google Scholar] [CrossRef]
- Chen, F.; Smith, P.W.; Dobson, P.J. Domain switching of PLZT 9/65/35 in ferroelectric electron emission. In Proceedings of the 2000 Eighth International Conference on Dielectric Materials, Measurements and Applications (IEE Conf. Publ. No. 473), Edinburgh, UK, 17–21 September 2000; pp. 182–187. [Google Scholar] [CrossRef]
- Kutnjak, Z.; Bobnar, V.; Filipič, C.; Levstik, A. Glassy properties of 9/65/35 PLZT ceramics. Ferroelectrics 2001, 257, 29–38. [Google Scholar] [CrossRef]
- Khodorov, A.; Gomes, M.J.M. Optical Properties of PLZT 9/65/35 Thin Films on ITO-Coated Glass Substrate. Mater. Sci. Forum 2006, 514–516, 193–197. [Google Scholar] [CrossRef]
- Somwan, S.; Ngamjarurojana, A.; Limpichaipanit, A. Dielectric, ferroelectric and induced strain behavior of PLZT 9/65/35 ceramics modified by Bi2O3 and CuO co-doping. Ceram. Int. 2016, 42, 10690–10696. [Google Scholar] [CrossRef]
- De Camargo, A.S.S.; Botero, E.R.; Nunes, L.A.O.; Lente, M.H.; Santos, I.A.; Andreeta, E.R.M.; Garcia, D.; Eiras, J.A. Ferroelectric ceramic materials as hosts of laser active ions: Structural, microstructural and spectroscopic characteristics. Ceramica 2004, 50, 368–377. [Google Scholar] [CrossRef]
- Plonska, M.; Dzik, J. Characterization of Lead Lanthanum Zirconate Titanate Ceramics Co-Doped with Lanthanide Ions. In Proceedings of the 7th Forum on New Materials—Part B 2016, Perugia, Italy, 5–9 June 2016. [Google Scholar] [CrossRef]
- Plonska, M.; Pisarski, W.A.; Pisarska, J. Luminescence Properties of Ytterbium Activated PLZT Ceramics. In Proceedings of the 7th Forum on New Materials—Part B 2016, Perugia, Italy, 5–9 June 2016. [Google Scholar] [CrossRef]
- De Queiroz, T.B.; Mohr, D.; Eckert, H.; de Camargo, A.S.S. Preparation and structural characterization of rare-earth doped lead lanthanum zirconate titanate ceramics. Solid State Sci. 2009, 11, 1363–1369. [Google Scholar] [CrossRef]
- Bai, Y. Photoresponsive Piezoelectrics. Front. Mater. 2021, 8, 636712. [Google Scholar] [CrossRef]
- Batra, V.; Kotru, S.; Varagas, M.; Ramana, C.V. Optical constants and band gap determination of Pb0.95La0.05Zr0.54Ti0.46O3 thin films using spectroscopic ellipsometry and UV–visible spectroscopy. Opt. Mater. 2015, 49, 123–128. [Google Scholar] [CrossRef]
- Dieke, G.H.; Crosswhite, H.M. The Spectra of the Doubly and Triply Ionized Rare Earths. Appl. Opt. 1963, 2, 675–686. [Google Scholar] [CrossRef]
- Shannigrahi, S.R.; Tripathy, S. Micro-Raman spectroscopic investigation of rare earth-modified lead zirconate titanate ceramics. Ceram. Int. 2007, 33, 595–600. [Google Scholar] [CrossRef]
- Płońska, M.; Adamczyk, M. Dielectric properties of neodymium-modified PLZT ceramics. Phase Trans. 2015, 88, 786–798. [Google Scholar] [CrossRef]
- Sternberg, A.; Dimza, V.; Sprogis, A.; Kapenieks, A.; Shebanov, L.; Grinvalds, G.; Rubulis, A.; Stumpe, R. Optical and dielectric properties of transparent PLZT ceramics with various defects. Ferroelectrics 1988, 80, 301–306. [Google Scholar] [CrossRef]
- Durruthy-Rodriguez, M.D.; Gervacio-Arciniega, J.J.; Hernandez-Garcia, M.; Yánez-Limon, J.M. Photoluminescence characteristics of soft PZT 53/47 ceramic doped at A and/or B sites. J. Adv. Ceram. 2018, 7, 109–116. [Google Scholar] [CrossRef]
- Plonska, M.; Pisarski, W.A. Excitation and emission of Pr3+:PLZT ceramics. Ceram. Int. 2016, 42, 17822–17826. [Google Scholar] [CrossRef]
- Huang, C.; Xu, J.; Fang, Z.; Ai, D.; Zhou, W.; Zhao, L.; Sun, J.; Wang, Q. Effect of preparation process on properties of PLZT (9/65/35) transparent ceramics. J. Alloys Compd. 2017, 723, 602–610. [Google Scholar] [CrossRef]
- Płońska, M.; Pisarski, W.A.; Wodecka-Duś, B.; Czekaj, D. The influence of fabrication conditions on the physical properties of PLZT:Nd3+ ceramics. Arch. Metall. Mater. 2013, 58, 1365–1369. [Google Scholar] [CrossRef]
- Patil, K.C.; Hedgde, M.S.; Rattan, T.; Aruna, S.T. Chemistry of Nanocrystalline Oxide Materials, Combustion Synthesis, Properties and Applications; World Scientific Publishing Co. Pte. Ltd.: Danvers, MA, USA, 2008. [Google Scholar] [CrossRef]
- Patil, K.C.; Aruna, S.T.; Mimani, T. Combustion synthesis: An update. Curr. Opin. Solid State Mater. Sci. 2002, 6, 507–512. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, V.S.; Tiwari, P.; Srivasrava, A.K.; Gupta, P.K. Effect of oxidant-to-fuel ratios on phase formation of PLZT powder; prepared by gel-combustion. J. Alloys Compd. 2011, 509, 4127–4131. [Google Scholar] [CrossRef]
- Bochenek, D. A Combination of Calcination and the spark plasma sintering method in multiferroic ceramic composite technology: Effects of process temperature and dwell time. Materials 2022, 15, 2524. [Google Scholar] [CrossRef]
- Kröger, F.; Vink, H. Relations between the Concentrations of Imperfections in Crystalline Solids. Solid State Phys. 1956, 3, 307–435. [Google Scholar] [CrossRef]
- Chang, T.I.; Huang, J.L.; Lin, H.P.; Wang, S.C.; Lu, H.H.; Wu, L.; Lin, J.F. Effect of drying temperature on structure, phase transformation of sol-gel derived lead zirconate titanate powders. J. Alloys Compd. 2006, 414, 224–229. [Google Scholar] [CrossRef]
- Scott, J.F.; Dawber, M. Oxygen-vacancy ordering as a fatigue mechanism in perovskite ferroelectrics. Appl. Phys. Lett. 2000, 76, 3801–3803. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data. Available online: https://www.icdd.com/?gclid=Cj0KCQjw6J-SBhCrARIsAH0yMZjpLPu8oJKSk86zbcA_VTtlnuyrD17YXNqXCudyEl9Hl9zZI7KhNzYaAtbcEALw_wcB (accessed on 10 March 2023).
- Mansour, S.; Eid, A.; El-Latif Abd, L.; Rashad, M.; Afifi, M.; Turner, J. Dielectric and piezoelectric performance of gadolinium-doped lead lanthanum zirconate titanate. Int. J. Appl. Ceram. Technol. 2018, 15, 766–774. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Fiaczyk, K.; Ramalho, J.F.C.B.; Sójka, M.; Carlos, L.D.; Zych, E. Widening the temperature range of luminescent thermometers through the intra- and interconfigurational transitions of Pr3+. Adv. Opt. Mater. 2018, 6, 1701318. [Google Scholar] [CrossRef]
- Boutinaud, P.; Mahiou, R.; Cavalli, E.; Bettinelli, M. Luminescence properties of Pr3+in titanates and vanadates: Towards a criterion to predict 3P0 emission quenching. Chem. Phys. Lett. 2006, 418, 185–188. [Google Scholar] [CrossRef]
- Zou, H.; Hui, X.; Wang, X.; Peng, D.; Li, J.; Li, Y.; Yao, X. Luminescent, dielectric, and ferroelectric properties of Pr doped Bi7Ti4NbO21 multifunctional ceramics. J. Appl. Phys. 2013, 114, 223103. [Google Scholar] [CrossRef]
- Górny, A.; Sołtys, M.; Pisarska, J.; Pisarski, W.A. Germanate glasses co-doped with Ce 3+ /Ln 3+ (Ln = Pr, Tb, Dy) for white light emitting diodes. Opt. Appl. 2019, 49, 383–391. [Google Scholar] [CrossRef]
- Available online: https://medium.com/hipster-color-science/a-beginners-guide-to-colorimetry-401f1830b65a (accessed on 5 May 2023).
- Tan, Q.; Viehland, D. Ac-field-dependent structure-property relationships in La-modified lead zirconate titanate: Induced relaxor behavior and domain breakdown in soft ferroelectrics. Phys. Rev. B 1996, 53, 14103. [Google Scholar] [CrossRef]
- Sato, Y.; Kanai, H.; Yamashita, Y. Grain size dependence of dielectric constant for modified (Pb0.63Ba0.37)(Zr0.7Ti0.3)O3 ceramic material. Jpn. J. Appl. Phys. 1994, 33, 1380–1384. [Google Scholar] [CrossRef]
- Uchino, K.; Nomura, S. Critical exponents of the dielectric constants in diffused-phase transition crystals. Ferroelectr. Lett. Sect. 1982, 44, 55–61. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Z.; Murakami, S.; Lu, Z.; Hall, D.A.; Sinclair, D.C.; Feteira, A.; Tan, X.; Jones, J.L.; Kleppe, A.K.; et al. Origin of the large electrostrain in BiFeO3-BaTiO3 based lead-free ceramics. J. Mater. Chem. A 2019, 7, 21254–21263. [Google Scholar] [CrossRef]
- Kim, S.; Miyauchi, R.; Sato, Y.; Nam, H.; Fujii, I.; Ueno, S.; Kuroiwa, Y.; Wada, S. Piezoelectric Actuation Mechanism Involving Extrinsic Nanodomain Dynamics in Lead-Free Piezoelectrics. Adv. Mater. 2023, 35, 2208717. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, X.; Ma, Y.; Zhang, X.; Lyu, J.; Wu, J. Deciphering the role of A-site ions of AZrO3-type dopants in (K, Na)NbO3 ceramics. Acta Mater. 2023, 254, 118997. [Google Scholar] [CrossRef]
- Picinin, A.; Lente, M.H.; Eiras, J.A.; Rino, J.P. Theoretical and experimental investigations of polarization switching in ferroelectric materials. Phys. Rev. B 2004, 69, 064117. [Google Scholar] [CrossRef]
- Bokov, A.A.; Ye, Z.-G. Recent Progress in Relaxor Ferroelectrics with Perovskite Structure. J. Mater. Sci. 2006, 41, 31–52. [Google Scholar] [CrossRef]
- Minhas, J.Z.; Hasan, A.M.; Yang, Y. Ferroelectric Materials Based Coupled Nanogenerators. Nanoenergy Adv. 2021, 1, 131–180. [Google Scholar] [CrossRef]




















| Chemical Formula | Designed |
|---|---|
| (Pb0.91La0.09)(Zr0.65Ti0.35)0.9775O3 | 9/65/35 PLZT:Pr3+0 |
| (Pb0.91La0.09)(Zr0.65Ti0.35)0.9775O3 + Pr3+0.1 wt.% | 9/65/35 PLZT:Pr3+0.1 |
| (Pb0.91La0.09)(Zr0.65Ti0.35)0.9775O3 + Pr3+0.3 wt.% | 9/65/35 PLZT:Pr3+0.3 |
| (Pb0.91La0.09)(Zr0.65Ti0.35)0.9775O3 + Pr3+0.5 wt.% | 9/65/35 PLZT:Pr3+0.5 |
| (Pb0.91La0.09)(Zr0.65Ti0.35)0.9775O3 + Pr3+1 wt.% | 9/65/35 PLZT:Pr3+1 |
| Compositions/Elements | The Theoretical Content of Elements (wt.%) | |||||
| Pb | La | Zr | Ti | O | Pr | |
| 9PLZT | 58.305 | 3.866 | 17.923 | 5.064 | 14.842 | --- |
| 9PLZT:Pr3+0.1 | 58.247 | 3.862 | 17.905 | 5.059 | 14.827 | 0.099 |
| 9PLZT:Pr3+0.3 | 58.131 | 3.854 | 17.869 | 5.049 | 14.797 | 0.299 |
| 9PLZT:Pr3+0.5 | 58.015 | 3.847 | 17.834 | 5.039 | 14.768 | 0.497 |
| 9PLZT:Pr3+1 | 57.728 | 3.821 | 17.746 | 5.014 | 14.695 | 0.996 |
| Compositions/Elements | Measured Content of Elements (wt.%) | |||||
| Pb | La | Zr | Ti | O | Pr | |
| 9PLZT | 59.006 | 3.780 | 17.292 | 5.087 | 14.835 | --- |
| 9PLZT:Pr3+0.1 | 58.462 | 3.659 | 17.979 | 5.065 | 14.749 | 0.086 |
| 9PLZT:Pr3+0.3 | 57.977 | 3.943 | 17.324 | 5.561 | 15.195 | 0.230 |
| 9PLZT:Pr3+0.5 | 58.128 | 3.772 | 17.689 | 5.080 | 14.790 | 0.541 |
| 9PLZT:Pr3+1 | 57.899 | 3.580 | 17.510 | 5.102 | 14.995 | 0.914 |
| Parameters/Samples | 9/65/35 PLZT | 9PLZT:Pr3+0.1 | 9PLZT:Pr3+0.3 | 9PLZT:Pr3+0.5 | 9PLZT:Pr3+1 |
|---|---|---|---|---|---|
| a0 (nm) | 4.074 | 4.041 | 4.052 | 4.063 | 4.079 |
| b0 (nm) | 4.074 | 4.041 | 4.052 | 4.063 | 4.079 |
| c0 (nm) | 4.074 | 4.041 | 4.052 | 4.063 | 4.079 |
| V × 10−30 (nm) | 67.62 | 65.99 | 66.53 | 67.07 | 67.53 |
| Rp (%) | 6.02 | 6.43 | 6.09 | 6.99 | 6.09 |
| Rwp (%) | 8.14 | 8.92 | 8.11 | 8.56 | 8.11 |
| Rexp (%) | 4.39 | 4.13 | 4.72 | 4.34 | 4.53 |
| ρ × 10−3 (g/cm3) | 7.66 | 7.21 | 7.18 | 7.52 | 7.38 |
| ρ/ρtheor (%) | 96 | 94 | 93 | 95 | 93 |
| Parameters/Samples | 9/65/35 PLZT | 9PLZT:Pr3+0.1 | 9PLZT:Pr3+0.3 | 9PLZT:Pr3+0.5 | 9PLZT:Pr3+1 |
|---|---|---|---|---|---|
| Δεm | 1305.86 | 2445.0 | 504.93 | 403.95 | 609.5 |
| ΔTm | 8.92 | 4.66 | 7.22 | 14.91 | 16.01 |
| εRT | 4889.64 | 2156.19 | 2332.80 | 2139.15 | 2138.10 |
| εm | 8692.69 | 9860.78 | 8400.07 | 6974.50 | 5025.47 |
| Tm (K) | 358.51 | 418.48 | 414.01 | 394.93 | 374.76 |
| T0 (K) | 380.16 | 446.89 | 454.70 | 442.48 | 396.81 |
| T’ (K) | 487.09 | 509.09 | 516.12 | 510.55 | 497.63 |
| k1 = εm/εRT | 1.78 | 4.57 | 3.60 | 3.26 | 2.35 |
| k2 = T’ − T0 (K) | 106.93 | 63.09 | 58.42 | 68.07 | 100.02 |
| k3 = T’ − Tm (K) | 128.58 | 90.61 | 102.11 | 115.62 | 122.87 |
| γ | 2.08 | 1.84 | 1.95 | 2.00 | 2.02 |
| Parameters/Samples | 9/65/35 PLZT | 9PLZT:Pr3+0.1 | 9PLZT:Pr3+0.3 | 9PLZT:Pr3+0.5 | 9PLZT:Pr3+1 |
|---|---|---|---|---|---|
| Ps (mC/cm2) | 29.09 | 18.07 | 22.15 | 26.59 | 28.30 |
| PR (mC/cm2) | 20.44 | 10.42 | 13.24 | 6.55 | 13.45 |
| EC (kV/mm) | 0.78 | 0.86 | 0.82 | 0.45 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płońska, M.; Plewa, J. Investigation of Praseodymium Ions Dopant on 9/65/35 PLZT Ceramics’ Behaviors, Prepared by the Gel-Combustion Route. Materials 2023, 16, 7498. https://doi.org/10.3390/ma16237498
Płońska M, Plewa J. Investigation of Praseodymium Ions Dopant on 9/65/35 PLZT Ceramics’ Behaviors, Prepared by the Gel-Combustion Route. Materials. 2023; 16(23):7498. https://doi.org/10.3390/ma16237498
Chicago/Turabian StylePłońska, Małgorzata, and Julian Plewa. 2023. "Investigation of Praseodymium Ions Dopant on 9/65/35 PLZT Ceramics’ Behaviors, Prepared by the Gel-Combustion Route" Materials 16, no. 23: 7498. https://doi.org/10.3390/ma16237498