The Recovery of Vanadium Pentoxide (V2O5) from Spent Catalyst Utilized in a Sulfuric Acid Production Plant in Jordan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Spent Vanadium Catalyst Samples
2.3. Extraction Methods
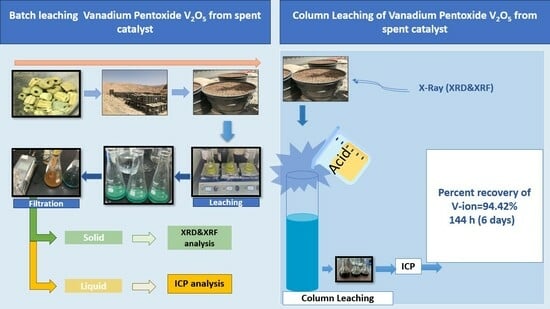
3. Results and Discussion
3.1. Characterization of the Spent Vanadium Catalyst
3.2. Effect of Experimental Conditions on Extraction Recovery
3.2.1. The Effect of Acid Type and Concentration on Extraction Recovery
3.2.2. The Effect of Oxalic Acid Concentration on Leaching Efficiency
3.2.3. The Effect of Stirring Rate
3.2.4. The Effect of Solid-to-Leachant Volume (S/L Ratio)
3.2.5. The Effect of Temperature on Vanadium Recovery from the Spent Catalyst
3.3. Column Leaching Experiments
3.4. Alkaline Leaching Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements, and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nasimifar, A.; Mehrabani, J. A review on the extraction of vanadium pentoxide from primary, secondary, and co-product sources. Int. J. Min. Geo. Eng. 2022, 56, 361–382. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q. Recovery of V2O5 from spent SCR catalyst by H2SO4-ascorbic acid leaching and chemical precipitation. J. Environ. Chem. Eng. 2022, 10, 108719. [Google Scholar] [CrossRef]
- Romanovskaia, E.; Romanovski, V.; Kwapinski, W.; Kurilo, I. Selective recovery of vanadium pentoxide from spent catalysts of sulfuric acid production: Sustainable approach. Hydrometallurgy 2021, 200, 105568. [Google Scholar] [CrossRef]
- Blum, R.-P.; Niehus, H.; Hucho, C.; Fortrie, R.; Ganduglia-Pirovano, M.V.; Sauer, J.; Shaikhutdinov, S.; Freund, H.-J. Surface Metal-Insulator Transition on a Vanadium Pentoxide (001) Single Crystal. Phys. Rev. Lett. 2007, 99, 226103. [Google Scholar] [CrossRef]
- Calderón, H.; Endara, D. Recovery of Vanadium from Acid and Basic Leach Solutions of Spent Vanadium Pentoxide Catalysts. J. Geol. Resour. Eng. 2015, 4, 213–218. [Google Scholar] [CrossRef]
- Ognyanova, A.; Ozturk, A.T.; Michelis, I.D.; Ferella, F.; Taglieri, G.; Akcil, A.; Vegliò, F. Metal extraction from spent sulfuric acid catalyst through alkaline and acidic leaching. Hydrometallurgy 2009, 100, 20–28. [Google Scholar] [CrossRef]
- Yang, B.; He, J.; Zhang, G.; Guo, J. (Eds.) Vanadium and its compounds. In Vanadium; Elsevier: Amsterdam, The Netherlands, 2021; pp. 9–32. [Google Scholar] [CrossRef]
- Wexler, P.; Judson, R.; de Marcellus, S.; de Knecht, J.; Leinala, E. Health effects of toxicants: Online knowledge support. Life Sci. 2016, 145, 284–293. [Google Scholar] [CrossRef]
- Mohanty, J.; Rath, P.C.; Bhattacharya, I.N.; Paramguru, R.K. The recovery of vanadium from spent catalyst: A case study. Miner. Process. Extr. Metall. 2011, 120, 56–60. [Google Scholar] [CrossRef]
- Bauer, G.; Güther, V.; Hess, H.; Otto, A.; Roidl, O.; Roller, H.; Sattelberger, S. Vanadium and Vanadium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Lim, Y.; Choi, J.; Yoo, K.; Lee, S.-H. Feasibility study on the use of magnetic susceptibility for recovery of vanadium component in magnetite. Geosystem Eng. 2022, 25, 280–284. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, H.; Liu, T.; Li, S.; Zhang, R.; Guo, Z. High efficient vanadium extraction from vanadium slag using an enhanced acid leaching-coprecipitation process. Sep. Purif. Technol. 2023, 304, 122319. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Qiu, S.; Zhou, X.; Li, C.; Deng, Z. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy 2010, 104, 193–200. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, C.; Fan, G.; Li, M.; Li, C.; Li, X. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferrous Met. Soc. China 2010, 20, s118–s122. [Google Scholar] [CrossRef]
- Jammulamadaka, H.; Pisupati, S.V. A Critical Review of Extraction Methods for Vanadium from Petcoke Ash. Fuels 2023, 4, 58–74. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Wang, H.; Miao, Y.; Wang, X.; Meng, S.; Feng, Q. Effect of dissolved components of malachite and calcite on surface properties and flotation behavior. Int. J. Miner. Metall. Mater. 2023, 30, 1297–1309. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, B.; Yi, Y.; Feng, Q.; Liu, D. Synergistic activation of smithsonite with copper-ammonium species for enhancing surface reactivity and xanthate adsorption. Int. J. Min. Sci. Technol. 2023, 33, 519–527. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Bedelova, Z.; Anarbekov, K.; Baikonurova, A.; Tuncuk, A. Recovery of vanadium from spent catalysts of sulfuric acid plant by using inorganic and organic acids: Laboratory and semi-pilot tests. Waste Manag. 2016, 49, 455–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Xiong, Z.; Gao, T.; Xiao, R.; Liu, P.; Liu, J.; Zhang, J. Photo- and thermo-catalytic mechanisms for elemental mercury removal by Ce doped commercial selective catalytic reduction catalyst (V2O5/TiO2). Chemosphere 2022, 287, 132336. [Google Scholar] [CrossRef]
- Tiwari, M.; Bajpai, S.; Dewangan, U.; Tamrakar, R. Suitability of leaching test methods for fly ash and slag: A review. J. Radiat. Res. Appl. Sci. 2015, 8, 523–537. [Google Scholar] [CrossRef]
- Odeh, F.; Abu-Dalo, M.; Albiss, B.; Ghannam, N.; Khalaf, A.; Amayreh, H.H.; Al Bawab, A. Coupling magnetite and goethite nanoparticles with sorbent materials for olive mill wastewater remediation. Emergent Mater. 2022, 5, 77–88. [Google Scholar] [CrossRef]
- Al Bawab, A.; Abu-Dalo, M.; Khalaf, A.; Abu-Dalo, D. Olive Mill Wastewater (OMW) Treatment Using Photocatalyst Media. Catalysts 2022, 12, 539–555. [Google Scholar] [CrossRef]
- Abbas, H.A.; Nasr, R.A.; Khalaf, A.; Bawab, A.A.; Jamil, T.S. Photocatalytic degradation of methylene blue dye by fluorite type Fe2Zr2−xWxO7 system under visible light irradiation. Ecotoxicol. Environ. Saf. 2020, 196, 110518. [Google Scholar] [CrossRef] [PubMed]
- Nasr, R.A.; Abbas, H.A.; Khalaf, A.; Bozeya, A.; Jamil, T.S. Nano-sized Ga2−xCuxZr2−xWxO7 for Malachite green decolorization under visible light. Desalination Water Treat. 2020, 183, 389–403. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Khalaf, A.; Abbas, H.A.; Nasr, R.A.; Jamil, T.S.; Al Bawab, A. Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation. Catalysts 2021, 11, 1473–1489. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Yi, H.; Zhang, Y.; Song, S.; Bao, S. Vanadium Transitions during Roasting-Leaching Process of Vanadium Extraction from Stone Coal. Minerals 2018, 8, 63. [Google Scholar] [CrossRef]
- Lee, M.; Le, M.N. Selective dissolution of vanadium (V) from spent petroleum catalysts by oxalic acid solution and its kinetic study. J. Min. Metall. Sect. B Metall. 2020, 56, 127–133. [Google Scholar] [CrossRef]
- Mazurek, K. Recovery of vanadium, potassium, and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation, and ion exchange processes. Hydrometallurgy 2013, 134, 26–31. [Google Scholar] [CrossRef]
- Irannajad, M.; Meshkini, M.; Azadmehr, A.R. Leaching of zinc from low grade oxide ore using organic acid. Physicochem. Probl. Miner. Process. 2013, 49, 547–555. [Google Scholar]
- Chen, Y.; Wang, Y.; Bai, Y.; Feng, M.; Zhou, F.; Lu, Y.; Mu, T. Mild and efficient recovery of lithium-ion battery cathode material by deep eutectic solvents with natural and cheap components. Green Chem. Eng. 2022, 4, 303–311. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Y.; He, D.; Yang, E.-H. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef]













| Constituents | Composition (w/w) % | Constituents | Composition (w/w) % | ||
|---|---|---|---|---|---|
| Active Vanadium Catalyst | Spent Vanadium Catalyst | Active Vanadium Catalyst | Spent Vanadium Catalyst | ||
| SiO2 | 64.3229 | 67.8672 | P2O5 | 0.2751 | 0.2184 |
| SO3 | 13.3427 | 11.4236 | MgO | 0.2198 | 0.1091 |
| K2O | 12.1145 | 11.0965 | MnO | 0.0132 | 0.0080 |
| V2O5 | 5.5119 | 5.3085 | NiO | 0.0081 | 0.0080 |
| Al2O3 | 1.5307 | 1.5182 | CuO | 0.0067 | 0.0049 |
| Fe2O3 | 1.1006 | 0.9865 | ZnO | 0.0062 | 0.0055 |
| Na2O | 0.8150 | 1.0137 | SrO | 0.0031 | 0.0040 |
| CaO | 0.7265 | 0.4279 | Rb2O | 0.0030 | N.D |
| Constituents | w/w% in CSC | Constituents | w/w% in CSC |
|---|---|---|---|
| SiO2 | 97.5526% | Na2O | 0.0669% |
| SO3 | 0.7799% | CaO | 0.0302% |
| K2O | 0.7623% | P2O5 | 0.0479% |
| V2O5 | 0.2964% | NiO | 0.0106% |
| Al2O3 | 0.3269% | CuO | 0.0028% |
| Fe2O3 | 0.1104% | MgO, MnO | N.D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Amayreh, H.H.; Khalaf, A.; Hawwari, M.I.; Hourani, M.K.; Al Bawab, A. The Recovery of Vanadium Pentoxide (V2O5) from Spent Catalyst Utilized in a Sulfuric Acid Production Plant in Jordan. Materials 2023, 16, 6503. https://doi.org/10.3390/ma16196503
Al Amayreh HH, Khalaf A, Hawwari MI, Hourani MK, Al Bawab A. The Recovery of Vanadium Pentoxide (V2O5) from Spent Catalyst Utilized in a Sulfuric Acid Production Plant in Jordan. Materials. 2023; 16(19):6503. https://doi.org/10.3390/ma16196503
Chicago/Turabian StyleAl Amayreh, Hiba H., Aya Khalaf, Majd I. Hawwari, Mohammed K. Hourani, and Abeer Al Bawab. 2023. "The Recovery of Vanadium Pentoxide (V2O5) from Spent Catalyst Utilized in a Sulfuric Acid Production Plant in Jordan" Materials 16, no. 19: 6503. https://doi.org/10.3390/ma16196503