Influence of the Chemical Composition of Ceria Conversion Coatings, Sealed in Solution of NaH2PO4 and Ca(NO3)2, on the Corrosion Behavior of Aluminum
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. SEM/EDS Studies
3.2. XRD Studies
3.3. XPS Studies
3.3.1. XPS of As-Deposited Samples
3.3.2. XPS of Studied Samples after Their Long-Term Exposure in CM
XPS of Studied Samples after Rp Tests
XPS of Studied Samples after OCP Tests
XPS of Studied Samples after Chronoamperometric Tests
3.4. Electrochemical Investigations
3.4.1. Polarization Curves
3.4.2. Eocp vs. Time Plotcharacterization
3.4.3. Chronoamperometric Investigations
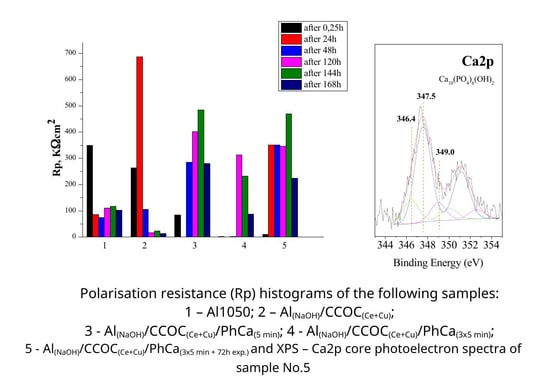
3.4.4. Investigations of Rp vs.Time of Exposure in CM
4. Conclusions
- There is substantial influence of the time and type of sealing post-treatment on the chemical composition and chemical state of the elements in the obtained and investigated systems. It is established there is a strong decrease of the concentration of Al2O3 and Ce2O3 components in the as-deposited CCOCs at the expense of the formation of insoluble phases: Ca5(PO4)3(OH); AlPO4; CePO4 and AlOOH (transformed in maximal stage of increase in Al(OH)3), as well as PO3−, compounds with Al and Ce, after their sealing post-treatment in mixed sodium phosphate and calcium nitrate solution;
- Based on the analyzed specific course of the potentiodynamic polarization dependences, changes in the anodic behavior of the aluminum substrate have been established, which are due to its modification with effective cathodic coatings;
- The comparison of these results with the changes of the concentrations of Ce3+, Al and P (also their respective oxides and phosphates) before and after exposure of the samples in CM show that the concentrations and chemical state of the Al, Ca, Ce and P (and their form as protective compounds) on the surface of the studied samples are directly related. In this case, it was found that the combination of oxide-phosphate compounds forming the conversion layers of system S5 has the best protective effect;
- Polarization investigations simultaneously showed that the combination of studied phosphate and ceria conversion layers are not only cathodic barrier coatings, but they also change the kinetics of the conjugated electrochemical reactions characterizing the corrosion process in Cl−-containing media, i.e., they determine the electrochemical protection of Al substrates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Commission Directive (EU) 2017/2096 of 15 November 2017 Amending Annex II to Directive 2000/53/EC of the European Parliament and of the Council on End-of Life Vehicles; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2019/2193 of 17 December 2019 Laying down Rules for the Calculation, Verification and Reporting of Data and Establishing Data Formats for the Purposes of Directive 2012/19/EU of the European Parliament and of the Council on Waste Electrical and Electronic Equipment (WEEE); European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Wernick, S.; Pinner, R. Surface Treatment and Finishing of Aluminium and Its Alloys; Robert Draper Ltd.: Sevenoaks, UK, 1956. [Google Scholar]
- Wernick, S.; Pinner, R.; Sheasby, P. The Surface Treatments of Aluminium and Its Alloys, 5th ed.; ASM International and Finishing Publications Ltd.: Metals Park, OH, USA, 1987. [Google Scholar]
- Harvey, T.G. Cerium-Based Conversion Coatings on Aluminium Alloys: A Process Review. Corros. Eng. Sci. Technol. 2013, 48, 248–269. [Google Scholar] [CrossRef]
- Twite, R.L.; Bierwagen, G.P. Review of Alternatives to Chromate for Corrosion Protection of Aluminum Aerospace Alloys. Prog. Org. Coatings 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Hinton, B. 2009 Frank Newman Speller Award Lecture: Prevention and Control of Corrosion in Aircraft Components—Changes over Four Decades. Corrosion 2010, 66, 0850011–08500115. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Hughes, A.E. Chromate and Chromate-Free Conversion Coatings. In ASM Handbook Vol. 13A: Corrosion: Fundamentals, Testing and Protection; ASM International: Detroit, MI, USA, 2003; pp. 720–735. [Google Scholar]
- Hinton, B.R.W.; Arnott, D.R.; Ryan, N.E. Cerium Conversion Coatings for the Corrosion Protection of Aluminium. Mater. Forum 1986, 9, 162–173. [Google Scholar]
- Hughes, A.E.; Taylor, R.J.; Hinton, B.R.W.; Wilson, L. XPS and SEM Characterization of Hydrated Cerium Oxide Conversion Coatings. Surf. Interface Anal. 1995, 23, 540–550. [Google Scholar] [CrossRef]
- Paussa, L.; Andreatta, F.; De Felicis, D.; Bemporad, E.; Fedrizzi, L. Investigation of AA2024-T3 Surfaces Modified by Cerium Compounds: A Localized Approach. Corros. Sci. 2014, 78, 215–222. [Google Scholar] [CrossRef]
- Eslami, M.; Fedel, M.; Speranza, G.; Deflorian, F.; Andersson, N.E.; Zanella, C. Study of Selective Deposition Mechanism of Cerium-Based Conversion Coating on Rheo-HPDC Aluminium-Silicon Alloys. Electrochim. Acta 2017, 255, 449–462. [Google Scholar] [CrossRef]
- Eslami, M.; Fedel, M.; Speranza, G.; Deflorian, F.; Zanella, C. Deposition and Characterization of Cerium-Based Conversion Coating on HPDC Low Si Content Aluminum Alloy. J. Electrochem. Soc. 2017, 164, C581. [Google Scholar] [CrossRef]
- Hassannejad, H.; Moghaddasi, M.; Saebnoori, E.; Baboukani, A.R. Microstructure, Deposition Mechanism and Corrosion Behavior of Nanostructured Cerium Oxide Conversion Coating Modified with Chitosan on AA2024 Aluminum Alloy. J. Alloys Compd. 2017, 725, 968–975. [Google Scholar] [CrossRef]
- Johnson, B.Y.; Edington, J.; Williams, A.; O’Keefe, M.J. Microstructural Characteristics of Cerium Oxide Conversion Coatings Obtained by Various Aqueous Deposition Methods. Mater. Charact. 2005, 54, 41–48. [Google Scholar] [CrossRef]
- Aldykewicz, A.J.; Isaacs, H.S.; Davenport, A.J. The Investigation of Cerium as a Cathodic Inhibitor for Aluminum-Copper Alloys. J. Electrochem. Soc. 1995, 142, 3342–3350. [Google Scholar] [CrossRef]
- Dabalà, M.; Armelao, L.; Buchberger, A.; Calliari, I. Cerium-Based Conversion Layers on Aluminum Alloys. Appl. Surf. Sci. 2001, 172, 312–322. [Google Scholar] [CrossRef]
- Arenas, M.A.; Bethencourt, M.; Botana, F.J.; De Damborenea, J.; Marcos, M. Inhibition of 5083 Aluminium Alloy and Galvanised Steel by Lanthanide Salts. Corros. Sci. 2001, 43, 157–170. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. On the Mixed Nature of Cerium Conversion Coatings. Mater. Corros. 2002, 53, 176–184. [Google Scholar] [CrossRef]
- Hughes, A.E.; Gorman, J.D.; Miller, P.R.; Sexton, B.A.; Paterson, P.J.K.; Taylor, R.J. Development of Cerium-Based Conversion Coatings on 2024-T3 Al Alloy after Rare-Earth Desmutting. Surf. Interface Anal. 2004, 36, 290–303. [Google Scholar] [CrossRef]
- Campestrini, P.; Terryn, H.; Hovestad, A.; Wit, J.H.W. de Formation of a Cerium-Based Conversion Coating on AA2024: Relationship with the Microstructure. Surf. Coat. Technol. 2004, 176, 365–381. [Google Scholar] [CrossRef]
- Davó, B.; De Damborenea, J.J. Use of Rare Earth Salts as Electrochemical Corrosion Inhibitors for an Al-Li-Cu (8090) Alloy in 3.56% NaCl. Electrochim. Acta 2004, 49, 4957–4965. [Google Scholar] [CrossRef]
- Danaee, I.; Zamanizadeh, H.R.; Fallahi, M.; Lotfi, B. The Effect of Surface Pre-Treatments on Corrosion Behavior of Cerium-Based Conversion Coatings on Al 7075-T6. Mater. Corros. 2014, 65, 815–819. [Google Scholar] [CrossRef]
- Johnson, B.Y.; Edington, J.; O’Keefe, M.J. Effect of Coating Parameters on the Microstructure of Cerium Oxide Conversion Coatings. Mater. Sci. Eng. A 2003, 361, 225–231. [Google Scholar] [CrossRef]
- You, S.; Jones, P.; Padwal, A.; Yu, P.; O’Keefe, M.; Fahrenholtz, W.; O’Keefe, T. Response of Nanocrystalline Cerium-Based Conversion Coatings on Al 2024-T3 to Chloride Environments. Mater. Lett. 2007, 61, 3778–3782. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Y. The Improvement of Corrosion Resistance of Ce Conversion Films on Aluminum Alloy by Phosphate Post-Treatment. Appl. Surf. Sci. 2008, 254, 4930–4935. [Google Scholar] [CrossRef]
- Kiyota, S.; Valdez, B.; Stoytcheva, M.; Zlatev, R.; Schorr, M. Electrochemical Study of Corrosion Behavior of Rare Earth Based Chemical Conversion Coating on Aerospace Aluminum Alloy. ECS Trans. 2009, 19, 115–123. [Google Scholar] [CrossRef]
- Andreeva, R.; Stoyanova, E.; Tsanev, A.; Stoychev, D. Influence of the Processes of Additional Phosphate Post-Treatment of Ceria Conversion Coatings Deposited on Al 1050 on Their Corrosion Protective Behavior. J. Phys. Conf. Ser. 2020, 1492, 012019. [Google Scholar] [CrossRef]
- Girginov, C.; Avramova, I.; Kozhukharov, S. Addition of Transition Elements to the Phosphate Sealing of Cerium Oxide Protective Primer Deposited on AA2024-T3 Alloy. J. Chem. Technol. Metall. 2016, 51, 705–714. [Google Scholar]
- Heller, D.K.; Fahrenholtz, W.G.; O’Keefe, M.J. Effect of Phosphate Source on Post-Treatment of Cerium-Based Conversion Coatings on Al 2024-T3. J. Electrochem. Soc. 2009, 156, C400–C406. [Google Scholar] [CrossRef]
- Heller, D.K. Phosphate Post-Treatment of Cerium-Based Conversion Coatings on Al 2024-T3. Ph.D. Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2010. [Google Scholar]
- Andreeva, R.; Stoyanova, E.; Tsanev, A.; Stoychev, D. Influence of the Surface Pre-Treatment of Aluminum on the Processes of Formation of Cerium Oxides Protective Films. J. Phys. Conf. Ser. 2016, 700, 012049. [Google Scholar] [CrossRef]
- Andreeva, R.; Stoyanova, E.; Stoychev, D. Study of the Chemical Formation of Cerium Oxide Conversion Layers on Aluminium AD-3. J. Int. Sci. Publ. Mater. Methods Technol. 2014, 8, 751–759. [Google Scholar]
- Stoyanova, E.; Stoychev, D. Electrochemical Aspects of the Immersion Treatment of Aluminium. J. Appl. Electrochem. 1997, 27, 685–690. [Google Scholar] [CrossRef]
- Andreeva, R.; Stoyanova, E.; Tsanev, A.; Datcheva, M.; Stoychev, D. On the Role of Pre-Treatment of Aluminum Substrate on Deposition of Cerium Based Conversion Layers and Their Corrosion-Protective Ability. Int. J. Electrochem. Sci. 2018, 13, 5333–5351. [Google Scholar] [CrossRef]
- Lee, H.S.; Kumar, A.; Mandal, S.; Singh, J.K.; Aslam, F.; Alyousef, R.; Albduljabbar, H. Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings. Coatings 2020, 10, 33. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Ismail, M.A. An Effective and Novel Pore Sealing Agent to Enhance the Corrosion Resistance Performance of Al Coating in Artificial Ocean Water. Sci. Rep. 2017, 7, 41935. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K. Influence of Calcium Nitrate on Morphology and Corrosion Characteristics of Ammonium Phosphate Treated Aluminum Coating Deposited by Arc Thermal Spraying Process. Corros. Sci. 2019, 146, 254–268. [Google Scholar] [CrossRef]
- Jeong, H.R.; Lee, H.S.; Jalalzai, P.; Kwon, S.J.; Singh, J.K.; Hussain, R.R.; Alyousef, R.; Alabduljabbar, H.; Aslam, F. Sodium Phosphate Post-Treatment on Al Coating: Morphological and Corrosion Study. J. Therm. Spray Technol. 2019, 28, 1511–1531. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Lukanova, R.; Stoyanova, E.; Damyanov, M.; Stoychev, D. Formation of Protective Films on Al in Electrolytes Containing No Cr6+ Ions. Bulg. Chem. Commun. 2008, 40, 340–347. [Google Scholar]
- Andreeva, R.; Stoyanova, E.; Tsanev, A.; Stoychev, D. XPS Characterisation of the Influence of Phosphate Post-Treatment of Chemically Deposited Ceria Protective Layers on Aluminum. Comptes Rendus L’Academie Bulg. Sci. 2019, 72, 1336–1342. [Google Scholar] [CrossRef]
- Tagai, H.; Aoki, H. Preparation of Synthetic Hydroxyapatite and Sintering of Apatite Ceramics: Mechanical Properties of Biomaterials; Hastings, G.W., Williams, D.F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1987; p. 213. [Google Scholar]
- Bouyer, E.; Gitzhofer, F.; Boulos, M.I. Morphological Study of Hydroxyapatite Nanocrystal Suspension. J. Mater. Sci. Mater. Med. 2000, 11, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Khajuria, A.; Sahu, J.K.; Swaminathan, J.; Kumar, R.; Bedi, R.; Albert, S.K. Phase Transformations and Numerical Modelling in Simulated HAZ of Nanostructured P91B Steel for High Temperature Applications. Appl. Nanosci. 2018, 8, 1669–1685. [Google Scholar] [CrossRef]
- Dalouji, V.; Rahimi, N.; Elahi, S.H. The Influence of Post-Annealing Temperatures on XPS, XZ Height of Nanoparticles, and Optical Properties of Cu–Al Doped ZnO Films. Mol. Cryst. Liq. Cryst. 2023, 758, 11–24. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Zhao, L.; Wu, A.; Li, A.; Dong, X.; Huang, H. AlPO4-Li3PO4 Dual Shell for Enhancing Interfacial Stability of Co-Free Li-Rich Mn-Based Cathode. Electrochim. Acta 2023, 462, 142664. [Google Scholar] [CrossRef]
- Sun, F.; Gao, W. Effect of Hydrochloric Acid on the Thermal Stability of Alpha-Aluminum Hydride. Int. J. Hydrog. Energy 2023, 48, 28998–29010. [Google Scholar] [CrossRef]
- Lie, J.; Shuwanto, H.; Abdullah, H.; Ismadji, S.; Warmadewanthi, I.D.A.A.; Soetaredjo, F.E. Fly Ash Electrodes Fabricated by an Acid-Assisted Subcritical Water Extraction Method for Supercapacitor Applications. New J. Chem. 2023, 47, 3802–3809. [Google Scholar] [CrossRef]
- Ivvala, J.; Arora, H.S.; Grewal, H.S. Towards Development of Sustainable Metallic Superhydrophobic Materials. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131047. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Yan, L.; Xing, Y.; Zhang, Z.; Zheng, Q.; Shen, J.; Zhao, X.; Wang, L. Stress-Dispersed Superstructure of Sn3(PO4)2@PC Derived from Programmable Assembly of Metal–Organic Framework as Long-Life Potassium/Sodium-Ion Batteries Anodes. Adv. Sci. 2023, 10, 2206587. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Zhou, Y.; Xie, Y.; Yang, J.; Wang, F. Formation of Protective Conversion Coating on Mg Surface by Inorganic Inhibitor. Corros. Sci. 2023, 215, 111044. [Google Scholar] [CrossRef]
- Yadav, P.; Rao, R.P.; Azeem, P.A. Optical and Structural Properties of Cost-Effective Nanostructured Calcium Titanate Blue Phosphor. Ceram. Int. 2023, 49, 6314–6323. [Google Scholar] [CrossRef]
- Eighmy, T.T.; Kinner, A.E.; Shaw, E.L.; Eusden, J.D.; Francis, C.A. Hydroxylapatite (Ca5(PO4)3OH) Characterization by XPS: An Environmentally Important Secondary Mineral. Surf. Sci. Spectra 1999, 6, 193–201. [Google Scholar] [CrossRef]
- McDowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the System Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37 o C. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 273–281. [Google Scholar] [CrossRef]
- Sharma, S.; Medpelli, D.; Chen, S.; Seo, D.K. Calcium-Modified Hierarchically Porous Aluminosilicate Geopolymer as a Highly Efficient Regenerable Catalyst for Biodiesel Production. RSC Adv. 2015, 5, 65454–65461. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, J.; Gao, Z.; Zhai, D.; Liu, R.; Qin, S.; Alam, M.K.; Sun, Z. Understanding the Complementary Resistive Switching in Egg Albumen-Based Single Sandwich Structure with Non-Inert Al Electrode. Mater. Res. Express 2023, 10, 056301. [Google Scholar] [CrossRef]
- Takakura, R.; Murakami, S.; Takigawa, R. Effect of Ar Fast Atom Beam Irradiation on Alpha-Al2O3 for Surface Activated Room Temperature Bonding. Jpn. J. Appl. Phys. 2023, 62, SG1046. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-Rich LiNi0.5Co0.2Mn0.3O2 Cathode Properties at High Operating Voltage by Double Coating Layer of Al2O3 and AlPO4. J. Alloys Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Chubar, N. The Influence of Sulfate on Selenate Sorption on Mg-Al-CO 3 Layered Double Hydroxides Prepared by Fine Inorganic Sol-Gel Synthesis Studied by X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2018, 459, 281–291. [Google Scholar] [CrossRef]
- Yu, H.; Dong, Q.; Jiao, Z.; Wang, T.; Ma, J.; Lu, G.; Bi, Y. Ion Exchange Synthesis of PAN/Ag3PO4 Core-Shell Nanofibers with Enhanced Photocatalytic Properties. J. Mater. Chem. A 2014, 2, 1668–1671. [Google Scholar] [CrossRef]
- Lee, Y.; Kang, J.; Ahn, J.; Ko, W.; Park, H.; Yoo, J.K.; Yoon, W.S.; Kim, J. Enhanced Conversion Reaction of Na-Cu-PO3 via Amorpholization and Carbon-Coating for Large Na Storage. Mater. Today Energy 2023, 35, 101325. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S. Enhanced Water Oxidation with Improved Stability by Aggregated RuO2-NaPO3 Core-Shell Nanostructures in Acidic Medium. Curr. Nanosci. 2017, 13, 333–341. [Google Scholar] [CrossRef]
- Plakhova, T.V.; Romanchuk, A.Y.; Yakunin, S.N.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.G.; Shuh, D.K.; Tyliszczak, T.; Shiryaev, A.A.; et al. Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling. J. Phys. Chem. C 2016, 120, 22615–22626. [Google Scholar] [CrossRef]
- Bolli, E.; Kaciulis, S.; Mezzi, A.; Montanari, R.; Varone, A. XPS Investigation of 5N Purity Al Thin Foils for MEMS Devices. Surf. Interface Anal. 2023, 55, 466–473. [Google Scholar] [CrossRef]
- Avramova, I.; Suzer, S.; Guergova, D.; Stoychev, D.; Stefanov, P. CeOx/Al2O3 Thin Films on Stainless Steel Substrate—Dynamical X-Ray Photoelectron Spectroscopy Investigations. Thin Solid Film 2013, 536, 63–67. [Google Scholar] [CrossRef]
- Zhuk, M.P. Theory of Corrosion and Protection of Metals; Metallurgy Publishing House: Moscow, Russia, 1976. (In Russian) [Google Scholar]
- Danilidis, I.; Hunter, J.; Scamans, G.M.; Sykes, J.M. Effects of Inorganic Additions on the Performance of Manganese-Based Conversion Treatments. Corros. Sci. 2007, 49, 1559–1569. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, G.; Wang, S.; Liu, Y.; Luo, H. A Chrome-Free Conversion Coating for Magnesium–Lithium Alloy by a Phosphate–Permanganate Solution. Surf. Coatings Technol. 2008, 202, 1825–1830. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]








| Type of Treatment of the Samples | Abbreviation of the Samples | ||
|---|---|---|---|
| in NaOH (1.5 M; 60 °C; 1 min) Solution | in 0.5 M CeCl3 × 7H2O + 1 × 10−5 M CuCl2 × 2H2O (25 °C; 2 h) | in 0.5 M NaH2PO4 + 0.1 M Ca(NO3)2 Solution (85 °C) | |
| S1 | YES | NO | NO |
| S2 | YES | YES | NO |
| S3 | YES | YES | 5 min. |
| S4 | YES | YES | 3 × 5 min. |
| S5 | YES | YES | 3 × 5 min + 72 h in humidity atmosphere |
| Sample | Al, wt.% | O, wt.% | Ce, wt.% | Ca, wt.% | P, wt.% | Cu, wt.% | Fe, wt.% | Cl, wt.% |
|---|---|---|---|---|---|---|---|---|
| S2 as-deposited | 86.09 | 10.4 | 3.14 | 0.37 | ||||
| after 168 h in 0.1 M NaCl | 93.68 | 4.68 | 1.64 | 0 | ||||
| S3 as-deposited | 80.83 | 12.34 | 6.55 | 0.28 | 0 | 0 | 0 | |
| after 168 h in 0.1 M NaCl | 88.87 | 8.67 | 1.33 | 0 | 1.13 | 0 | 0 | 0 |
| S4 as-deposited | 63.97 | 25.57 | 2.58 | 0.95 | 6.48 | 0.45 | 0 | |
| after 168 h in 0.1 M NaCl | 91.38 | 7.73 | 0 | 0 | 0.89 | 0 | 0 | 0 |
| S5 as-deposited | 68.94 | 22.64 | 2.12 | 0.84 | 5.1 | 0 | 0.35 | 0.37 |
| after 168 h in 0.1 M NaCl | 94.48 | 5.15 | 0 | 0 | 0 | 0 | 0 |
| Sample | Al, at.% | O, at.% | Ce, at.% | Ca, at.% | P, at.% | Cu, at.% | Na, at.% | N, at.% |
|---|---|---|---|---|---|---|---|---|
| S2 as-deposited | 11.0 | 70.5 | 15.8 | 2.7 | ||||
| after 168 h Rp | 20.5 | 76.7 | 2.4 | 0.4 | ||||
| after 168 h Chronoamperometry | 26.0 | 74.0 | ||||||
| after 168 h OCP | 16.2 | 78.1 | 5.7 | |||||
| S3 as-deposited | 17.1 | 61.8 | 3.84 | 2.3 | 14.3 | 0.6 | ||
| after 168 h Rp | 27.7 | 71.2 | 0.7 | 0.4 | ||||
| after 168 h Chronoamperometry | 30.5 | 69.5 | ||||||
| S4 as-deposited | 34.7 | 53.4 | 0.4 | 8.8 | 1.4 | 0.7 | 0.6 | |
| after 168 h Rp | 29.9 | 62.6 | 4.4 | 3.0 | ||||
| after 168 h OCP | 24.2 | 65.2 | 0.5 | 0.2 | 6.8 | 3.0 | ||
| S5 as-deposited | 19.6 | 65.1 | 0.5 | 1.7 | 11.6 | 0.6 | 1.0 | |
| after 168 h Rp | 28.2 | 68.4 | 3.5 | |||||
| after 168 h OCP | 24.0 | 63.4 | 1.1 | 0.6 | 8.2 | 2.7 |
| Sample | Ecor, V | icor, A.cm−2 | ipass, A.cm−2 | Er.p.s., V |
|---|---|---|---|---|
| S1 | −1.705 | 9.8 × 10−5 | 5.8 × 10−4 | −1.425 ÷ −0.587 |
| S2 | −1.472 | 8.1 × 10−5 | 3.4 × 10−4 | −1.263 ÷ −0.480 |
| S3 | −1.550 | 5.7 × 10−5 | 5.5 × 10−4 | −1.360 ÷ −0.585 |
| S4 | −1.553 | 4.5 × 10−5 | 3.6 × 10−4 | −1.388 ÷ −0.593 |
| S5 | −1.682 | 1.7 × 10−5 | 1.0 × 10−4 | −1.537 ÷ −0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsanev, A.; Andreeva, R.; Stoychev, D. Influence of the Chemical Composition of Ceria Conversion Coatings, Sealed in Solution of NaH2PO4 and Ca(NO3)2, on the Corrosion Behavior of Aluminum. Materials 2023, 16, 6499. https://doi.org/10.3390/ma16196499
Tsanev A, Andreeva R, Stoychev D. Influence of the Chemical Composition of Ceria Conversion Coatings, Sealed in Solution of NaH2PO4 and Ca(NO3)2, on the Corrosion Behavior of Aluminum. Materials. 2023; 16(19):6499. https://doi.org/10.3390/ma16196499
Chicago/Turabian StyleTsanev, Aleksandar, Reni Andreeva, and Dimitar Stoychev. 2023. "Influence of the Chemical Composition of Ceria Conversion Coatings, Sealed in Solution of NaH2PO4 and Ca(NO3)2, on the Corrosion Behavior of Aluminum" Materials 16, no. 19: 6499. https://doi.org/10.3390/ma16196499