Recent Advances on Porous Siliceous Materials Derived from Waste
Abstract
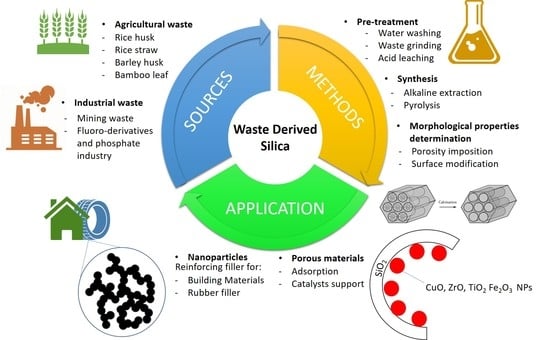
:1. Introduction
2. Silica from Industrial Production Waste or End-of-Life Products
2.1. Agricultural Waste
2.2. Industrial Waste
2.3. Work-Up Procedures for Agricultural and Industrial Waste
2.4. Silica Recovery from Hexafluorosilic Acid
3. Application of Waste-Derived Silica
3.1. Industrial Applications of Waste-Derived Silica
3.2. Porous Waste-Derived Silica
4. Conclusions
- (I)
- The majority of the case studies relies on the use of agricultural (e.g., rice husk) and industrial waste (primarily deriving from mills and mining operations).
- (II)
- The recent trend is the valorization of secondary products as a potential source of silica. In this context, FSA is a promising candidate as it was recently recognized as being a valuable source of Si-containing commodities. Even if the production of SiO2 from FSA is still a challenging route, this process potentially guarantees the improvement of environmental issues related to FSA disposal, which is mostly performed by direct neutralization into the sea (with environmental consequences easy to assume).
5. Future Perspectives
- (I)
- The difficulties of reaching uniform experimental and procedural parameters. This criticality is typically associated with processes based on the re-use of agricultural wastes. Biomasses are characterized by showing an intrinsic variability in terms of the type and origin of the biomass, seasonality, and purification processes used for the recovery of the given waste, which usually contains a high content of not easily removable impurities.
- (II)
- The second concern, instead, is cross-sectional for all the alternative processes discussed here, which are the environmental and handling issues related to the use of strong acids and bases. This last assumption is a clear example of how the valorization of sustainable resources for producing high-added-value chemicals may not be a sustainable alternative if the adopted alternative process requires the use of hazardous procedures analogous to traditional procedures. Therefore, a critical balance between the benefits and drawbacks is always mandatory.
- (III)
- The last concern is relative to the criticalities associated with the templating procedure necessary for obtaining porous SiO2, which remains analogous to the traditional routes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BR | butadiene rubber. |
| CTAB | cetyltrimetilammonium bromide. |
| FSA | hexafluorosilic acid. |
| GO | graphene oxide. |
| MB | methylene blue dye. |
| NPs | nanoparticles. |
| PEG | polyethylene glycol. |
| RT | room temperature. |
| SSA | specific surface area. |
| SBR | styrene butadiene rubber. |
| TEOS | tetraethyl orthosilicate. |
| TMSO | tetramethyl orthosilicate |
References
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0098 (accessed on 1 June 2023).
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Zhang, Z.; Malik, M.Z.; Khan, A.; Ali, N.; Malik, S.; Bilal, M. Environmental Impacts of Hazardous Waste, and Management Strategies to Reconcile Circular Economy and Eco-Sustainability. Sci. Total Environ. 2022, 807, 150856. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Wang, X.; Liu, P.; Li, J.; Liu, G.; Wang, K.; Li, M.; Zhong, Z.; Xu, J.; et al. Catalytic Conversion of Rubber Wastes to Produce Aromatic Hydrocarbons over USY Zeolites: Effect of SiO2/Al2O3 Mole Ratio. Energy Convers. Manag. 2019, 197, 111857. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Sun, P.; Xue, H.; Zhang, L.; Chen, J.; Zhang, H.; Zhu, W. Using Waste to Treat Waste: Red Mud Induced Hierarchical Porous γ-AlOOH and γ-Al2O3 Microspheres as Superior Pd Support for Catalytic Reduction of 4-Nitrophenol. Particuology 2023, 73, 59–67. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Dixit, A.; Bajaj, R. Development of Bio-Composite Material by Utilizing Chrome Containing Leather Waste with Al2O3 Ceramic Particles. Mater. Res. Express 2019, 6, 105105. [Google Scholar] [CrossRef]
- Gupta, J.; Jethoo, A.S.; Lata, N. Utilization of Waste Glass for Enhancement of Chemical Properties of Concrete. Nat. Environ. Pollut. Technol. 2023, 22, 237–244. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, Y.G.; Song, Y.; Kim, H.Y.; Nguyen, D.T.; Bae, H.J. Converting Textile Waste into Value-Added Chemicals: An Integrated Bio-Refinery Process. Environ. Sci. Ecotechnol. 2023, 15, 100238. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of Biomass Waste into High Value Chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E.; Perathoner, S. Waste to Chemicals for a Circular Economy. Chem. Eur. J. 2018, 24, 11831–11839. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Feng, A.; McCoy, B.J.; Munir, Z.A.; Cagliostro, D.E. Water Adsorption and Desorption Kinetics on Silica Insulation. J. Colloid Interface Sci. 1996, 180, 276–284. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Golafshani, E.M.; Behnood, A. Estimating the Optimal Mix Design of Silica Fume Concrete Using Biogeography-Based Programming. Cem. Concr. Compos. 2019, 96, 95–105. [Google Scholar] [CrossRef]
- Wolff, S.; Wang, M.J. Filler-Elastomer Interactions. Part IV. The Effect of the Surface Energies of Fillers on Elastomer Reinforcement. Rubber Chem. Technol. 1992, 65, 329–342. [Google Scholar] [CrossRef]
- Govardhane, S.; Shende, P. Integration of Green Nanotechnology with Silica for Corrosion Inhibition. Corros. Rev. 2021, 39, 211–218. [Google Scholar] [CrossRef]
- Mirabdolazimi, S.M.; Kargari, A.H.; Pakenari, M.M. New Achievement in Moisture Sensitivity of Nano-Silica Modified Asphalt Mixture with a Combined Effect of Bitumen Type and Traffic Condition. Int. J. Pavement Res. Technol. 2021, 14, 105–115. [Google Scholar] [CrossRef]
- Goyal, P.; Purdue, M.J.; Farooq, S. Adsorption and Diffusion of Moisture and Wet Flue Gas on Silica Gel. Chem. Eng. Sci. 2020, 227, 8891–8913. [Google Scholar] [CrossRef]
- Wang, W.; Dong, W.; Tian, G.; Sun, L.; Wang, Q.; Hui, A.; Mu, B.; Wang, A. Highly Efficient Self-Template Synthesis of Porous Silica Nanorods from Natural Palygorskite. Powder Technol. 2019, 354, 1–10. [Google Scholar] [CrossRef]
- Nisticò, R.; Magnacca, G.; Antonietti, M.; Fechler, N. “Salted Silica”: Sol-Gel Chemistry of Silica under Hypersaline Conditions. Z. Anorg. Allg. Chem. 2014, 640, 582–587. [Google Scholar] [CrossRef]
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent Progress in Hollow Silica: Template Synthesis, Morphologies and Applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]
- Liu, S.; Dun, C.; Wei, J.; An, L.; Ren, S.; Urban, J.J.; Swihart, M.T. Creation of Hollow Silica-Fiberglass Soft Ceramics for Thermal Insulation. Chem. Eng. J. 2023, 454, 140134. [Google Scholar] [CrossRef]
- Wróblewska, A.; Makuch, E.; Retajczyk, M.; Sreńscek-Nazzal, J.; Koren, Z.C.; Michalkiewicz, B. Synthesis, Characterization and Application of the SBA-16 Catalyst Modified with Titanium(IV) Chloride in the Eugenol Isomerization. Microporous Mesoporous Mater. 2021, 311, 110685. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Zhuang, C.; Li, L.; Liu, Y.; Ban, C.; Liu, X. Boron-Assisted Growth of Silica Nanowire Arrays and Silica Microflowers for Bendable Capacitor Application. Chin. Chem. Lett. 2018, 29, 954–958. [Google Scholar] [CrossRef]
- Scotti, R.; Conzatti, L.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Stagnaro, P.; Susanna, A.; Tadiello, L.; Morazzoni, F. Shape Controlled Spherical (0D) and Rod-like (1D) Silica Nanoparticles in Silica/Styrene Butadiene Rubber Nanocomposites: Role of the Particle Morphology on the Filler Reinforcing Effect. Polymer 2014, 55, 1497–1506. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Nisticò, R.; Magnacca, G.; Scalarone, D. Packed Hybrid Silica Nanoparticles as Sorbents with Thermo-Switchable Surface Chemistry and Pore Size for Fast Extraction of Environmental Pollutants. RSC Adv. 2018, 8, 1246–1254. [Google Scholar] [CrossRef]
- Chen, G.; Luo, H.; Kang, C.; Luo, G.; Zhou, Y.; Pan, G. Application of Surface Analysis in Study on Removal Mechanism and Abrasive Selection during Fused Silica Chemical Mechanical Polishing. Surf. Interface Anal. 2019, 51, 576–583. [Google Scholar] [CrossRef]
- Zainal, N.S.; Mohamad, Z.; Mustapa, M.S.; Badarulzaman, N.A.; Zulkifli, A.Z. The Ability of Crystalline and Amorphous Silica from Rice Husk Ash to Perform Quality Hardness for Ceramic Water Filtration Membrane. Int. J. Integr. Eng. 2019, 11, 229–235. [Google Scholar] [CrossRef]
- Hu, P.; Ai, D.; Jiang, X.; Zhang, X. Fabrication of Hollow Silica Nanosphere and Its Application for Thermal Insulation Coating. J. Thermoplast. Compos. Mater. 2020, 33, 198–213. [Google Scholar] [CrossRef]
- Tsukada, S.; Nakanishi, Y.; Hamada, T.; Okada, K.; Mineoi, S.; Ohshita, J. Ethylene-Bridged Polysilsesquioxane/Hollow Silica Particle Hybrid Film for Thermal Insulation Material. RSC Adv. 2021, 11, 24968–24975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, F.; Xiao, M.; Zhang, Z.; Wei, J.; Hu, J.; Yu, Q. TEOS and Na2SiO3 as Silica Sources: Study of Synthesis and Characterization of Hollow Silica Nanospheres as Nano Thermal Insulation Materials. Appl. Nanosci. 2020, 10, 1833–1844. [Google Scholar] [CrossRef]
- Susanna, A.; Armelao, L.; Callone, E.; Dirè, S.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Morazzoni, F.; Scotti, R. ZnO Nanoparticles Anchored to Silica Filler. A Curing Accelerator for Isoprene Rubber Composites. Chem. Eng. J. 2015, 275, 245–252. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujii, S.; Shitajima, K.; Fujiwara, K.; Hikasa, S.; Nakamura, Y. Soft Polymer-Silica Nanocomposite Particles as Filler for Pressure-Sensitive Adhesives. Polymer 2015, 70, 77–87. [Google Scholar] [CrossRef]
- Mazumder, A.; Chanda, J.; Bhattacharyya, S.; Dasgupta, S.; Mukhopadhyay, R.; Bhowmick, A.K. Improved Tire Tread Compounds Using Functionalized Styrene Butadiene Rubber-Silica Filler/Hybrid Filler Systems. J. Appl. Polym. Sci. 2021, 138, 51236. [Google Scholar] [CrossRef]
- Bhunia, S.; Sen, R.; Koner, S. Anchoring of Palladium(II) in Chemically Modified Mesoporous Silica: An Efficient Heterogeneous Catalyst for Suzuki Cross-Coupling Reaction. Inorg. Chim. Acta 2010, 363, 3993–3999. [Google Scholar] [CrossRef]
- Hyde, E.D.E.R.; Seyfaee, A.; Neville, F.; Moreno-Atanasio, R. Colloidal Silica Particle Synthesis and Future Industrial Manufacturing Pathways: A Review. Ind. Eng. Chem. Res. 2016, 55, 8891–8913. [Google Scholar] [CrossRef]
- Zhan, G.; Zeng, H.C. Integrated Nanocatalysts with Mesoporous Silica/Silicate and Microporous MOF Materials. Coord. Chem. Rev. 2016, 320–321, 181–192. [Google Scholar] [CrossRef]
- To, P.K.; Ma, H.T.; Nguyen Hoang, L.; Nguyen, T.T. Nitrate Removal from Waste-Water Using Silica Nanoparticles. J. Chem. 2020, 2020, 8861423. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Ram, R.N. Removal of Basic Dye from Waste-Water Using Silica as Adsorbent. Environ. Pollut. 1992, 77, 79–86. [Google Scholar] [CrossRef]
- Elkady, H.M.; Yasien, A.M.; Elfeky, M.S.; Serag, M.E. Assessment of Mechanical Strength of Nano Silica Concrete (NSC) Subjected to Elevated Temperatures. J. Struct. Fire Eng. 2019, 10, 90–109. [Google Scholar] [CrossRef]
- Khan, K.; Ahmad, W.; Amin, M.N.; Nazar, S. Nano-Silica-Modified Concrete: A Bibliographic Analysis and Comprehensive Review of Material Properties. Nanomaterials 2022, 12, 1989. [Google Scholar] [CrossRef] [PubMed]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of Quercetin Flavonoid in MCM-41 Mesoporous Silica: Positive Effect of Surface Functionalization. J. Colloid Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Prabakaran, A.; Agrawal, M.; Alexander, A. Biomedical Applications of Mesoporous Silica Nanoparticles as a Drug Delivery Carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Kruk, M. Synthesis of Large-Pore SBA-15 Silica from Tetramethyl Orthosilicate Using Triisopropylbenzene as Micelle Expander. Colloids Surf. A Physicochem. Eng. Asp. 2010, 357, 91–96. [Google Scholar] [CrossRef]
- Ernawati, L.; Balgis, R.; Ogi, T.; Okuyama, K. Tunable Synthesis of Mesoporous Silica Particles with Unique Radially Oriented Pore Structures from Tetramethyl Orthosilicate via Oil-Water Emulsion Process. Langmuir 2017, 33, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Qu, W.; Cheng, J.; Lei, Y.; Liu, M.; Wang, D. Epoxy/Nano-SiO2 Anticorrosion Coatings Synthesized by Different Molar Ratio of Tetraethyl Orthosilicate (TEOS) and Tetramethyl Orthosilicate (TMOS). Int. J. Electrochem. Sci. 2019, 14, 11641–11650. [Google Scholar] [CrossRef]
- Zarei, V.; Mirzaasadi, M.; Davarpanah, A.; Nasiri, A.; Valizadeh, M.; Hosseini, M.J.S. Environmental Method for Synthesizing Amorphous Silica Oxide Nanoparticles from a Natural Material. Processes 2021, 9, 334. [Google Scholar] [CrossRef]
- Di Credico, B.; Manzini, E.; Viganò, L.; Canevali, C.; D’Arienzo, M.; Mostoni, S.; Nisticò, R.; Scotti, R. Silica nanoparticles self-assembly process in polymer composites: Towards advanced materials. Ceram. Int. 2023, 49, 26165–26181. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Q.; Hao, W.; Ding, C.; Wang, Y.; Zeng, H. Controlling of Fumed Silica Particle Size Uniform Production Process Based on Burner Fluid Dynamic Simulation. Ind. Eng. Chem. Res. 2022, 61, 7235–7244. [Google Scholar] [CrossRef]
- Toshiro, D.; Marinescu, I.D.; Kurokawa, S. Advances in CMP Polishing Technologies; William Andrew: Oxford, UK, 2012; pp. 113–228. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Flame Aerosol Synthesis of Ceramic Powders. Prog. Energy Combust. Sci. 1998, 24, 197–219. [Google Scholar] [CrossRef]
- Tillman, L.; Voskanyan, A.; Navrotsky, A. Synthesis of Mesoporous Silica Using a Mineral Silica Source. J. Am. Ceram. Soc. 2023, 106, 1993–1999. [Google Scholar] [CrossRef]
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-Derived Silica Nanoparticles and Composites for Biosensors, Bioimaging, Drug Delivery and Supercapacitors: A Review. Environ. Chem. Lett. 2021, 19, 1667–1691. [Google Scholar] [CrossRef] [PubMed]
- Silviana, S.; Sanyoto, G.J.; Darmawan, A.; Sutanto, H. Geothermal Silica Waste as Sustainable Amorphous Silica Source for the Synthesis of Silica Xerogels. Rasayan J. Chem. 2020, 13, 1692–1700. [Google Scholar] [CrossRef]
- Saputri, Y.P.; Priyambodo, E. Adsorption of Cu(II) and Zn(II) on Liquid Waste of Electroplating Home Industry Using Silica Based Adsorbent Prepared from Glass Waste. Indones. J. Chem. Environ. 2020, 2, 7–12. [Google Scholar] [CrossRef]
- Liou, T.H.; Jheng, J.Y. Synthesis of High-Quality Ordered Mesoporous Carbons Using a Sustainable Way from Recycling of E-Waste as a Silica Template Source. ACS Sustain. Chem. Eng. 2018, 6, 6507–6517. [Google Scholar] [CrossRef]
- Yadav, M.; Dwibedi, V.; Sharma, S.; George, N. Biogenic Silica Nanoparticles from Agro-Waste: Properties, Mechanism of Extraction and Applications in Environmental Sustainability. J. Environ. Chem. Eng. 2022, 10, 108550. [Google Scholar] [CrossRef]
- Sapawe, N.; Hanafi, M.F. Production of Silica from Agricultural Waste. Arch. Org. Inorg. Chem. Sci. 2018, 3, 342–343. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, J.; Mridha, D.; Sarkar, J.; Orasugh, J.T.; Gangopadhyay, B.; Chattopadhyay, D.; Roychowdhury, T.; Acharya, K. Synthesis of Nanosilica from Agricultural Wastes and Its Multifaceted Applications: A Review. Biocatal. Agric. Biotechnol. 2021, 37, 102175. [Google Scholar] [CrossRef]
- Laad, M.; Datkhile, R. Effect of Process Parameters on the Microstructural Properties of Silica Extracted from Agro-Waste. Silicon 2021, 13, 1433–1439. [Google Scholar] [CrossRef]
- Vacca, M.A.; Cara, C.; Mameli, V.; Sanna Angotzi, M.; Scorciapino, M.A.; Cutrufello, M.G.; Musinu, A.; Tyrpekl, V.; Pala, L.; Cannas, C. Hexafluorosilicic Acid (FSA): From Hazardous Waste to Precious Resource in Obtaining High Value-Added Mesostructured Silica. ACS Sustain. Chem. Eng. 2020, 8, 14286–14300. [Google Scholar] [CrossRef]
- Salinas-Rodriguez, E.; Flores-Badillo, J.; Hernandez-Avila, J.; Cerecedo-Saenz, E.; Gutierrez-Amador, M.d.P.; Jeldres, R.I.; Toro, N. Assessment of Silica Recovery from Metallurgical Mining Waste, by Means of Column Flotation. Metals 2020, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yu, J.; Ma, C.; Bikane, K.; Sun, L. Catalytic Performance and Debromination of Fe–Ni Bimetallic MCM-41 Catalyst for the Two-Stage Pyrolysis of Waste Computer Casing Plastic. Chemosphere 2020, 248, 125964. [Google Scholar] [CrossRef] [PubMed]
- Sirinwaranon, P.; Sricharoenchaikul, V.; Atong, D. Catalytic Performance of Co, Fe on MCM-41 Synthesized from Illite Waste for Gasification of Torrefied Cassava Rhizome. Energy Rep. 2021, 7, 149–162. [Google Scholar] [CrossRef]
- Akhayere, E.; Kavaz, D.; Vaseashta, A. Efficacy Studies of Silica Nanoparticles Synthesized Using Agricultural Waste for Mitigating Waterborne Contaminants. Appl. Sci. 2022, 12, 9279. [Google Scholar] [CrossRef]
- September, L.A.; Kheswa, N.; Seroka, N.S.; Khotseng, L. Green Synthesis of Silica and Silicon from Agricultural Residue Sugarcane Bagasse Ash—A Mini Review. RSC Adv. 2023, 13, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture: Trends and Challenges. Available online: https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 30 May 2023).
- Babla, M.; Katwal, U.; Yong, M.T.; Jahandari, S.; Rahme, M.; Chen, Z.H.; Tao, Z. Value-Added Products as Soil Conditioners for Sustainable Agriculture. Resour. Conserv. Recycl. 2022, 178, 106079. [Google Scholar] [CrossRef]
- Patsios, S.I.; Kontogiannopoulos, K.N.; Banias, G.F. Environmental Impact Assessment in Agri-Production: A Comparative Study of Olive Oil Production in Two European Countries. In Bio-Economy and Agri-Production: Concepts and Evidence; Bochtis, D., Achillas, C., Banias, G., Lampridi, M., Eds.; Academic Press: London, UK, 2021; pp. 83–116. [Google Scholar] [CrossRef]
- Aneja, V.P.; Schlesinger, W.H.; Erisman, J.W. Effects of Agriculture upon the Air Quality and Climate: Research, Policy, and Regulations. Environ. Sci. Technol. 2009, 43, 4234–4240. [Google Scholar] [CrossRef] [Green Version]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Martire, D.O. Biowaste-Derived Substances as a Tool for Obtaining Magnet-Sensitive Materials for Environmental Applications in Wastewater Treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Shah, B.A.; Patel, A.V.; Bagia, M.I.; Shah, A.V. Green Approach towards the Synthesis of MCM-41 from Siliceous Sugar Industry Waste. Int. J. Appl. Chem. 2017, 13, 497–514. [Google Scholar]
- Arumugam, A.; Karuppasamy, G.; Jegadeesan, G.B. Synthesis of Mesoporous Materials from Bamboo Leaf Ash and Catalytic Properties of Immobilized Lipase for Hydrolysis of Rubber Seed Oil. Mater. Lett. 2018, 225, 113–116. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Zhang, C.; Shi, Y.; Jin, G.; Yuan, S. Preparation of Nano-Silica Materials: The Concept from Wheat Straw. J. Non Cryst. Solids 2010, 356, 2781–2785. [Google Scholar] [CrossRef]
- Ramasamy, S.P.; Veeraswamy, D.; Ettiyagounder, P.; Arunachalam, L.; Devaraj, S.S.; Krishna, K.; Oumabady, S.; Sakrabani, R. New Insights Into Method Development and Characterization of Amorphous Silica From Wheat Straw. Silicon, 2023; in press. [Google Scholar] [CrossRef]
- Adigun, B.O.; Jegede, F.I.; Tunmilayo Sanya, O. Advanced Materials Development from Corncob Ash for Economic Sustainability. Int. J. Ceram. Eng. Sci. 2020, 2, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Wardhani, G.A.P.K.; Nurlela, N.; Azizah, M. Silica Content and Structure from Corncob Ash with Various Acid Treatment (HCl, HBr, and Citric Acid). Molekul 2017, 12, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhao, P.; Shao, Q. Porous Silica and Carbon Derived Materials from Rice Husk Pyrolysis Char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Park, J.Y.; Gu, Y.M.; Park, S.Y.; Hwang, E.T.; Sang, B.I.; Chun, J.; Lee, J.H. Two-Stage Continuous Process for the Extraction of Silica from Rice Husk Using Attrition Ball Milling and Alkaline Leaching Methods. Sustainability 2021, 13, 7350. [Google Scholar] [CrossRef]
- Purwaningsih, H.; Ervianto, Y.; Pratiwi, V.M.; Susanti, D.; Purniawan, A. Effect of Cetyl Trimethyl Ammonium Bromide as Template of Mesoporous Silica MCM-41 from Rice Husk by Sol-Gel Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012051. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Pasymi, P.; Bindar, Y. An Appropriate Acid Leaching Sequence in Rice Husk Ash Extraction to Enhance the Produced Green Silica Quality for Sustainable Industrial Silica Gel Purpose. J. Taiwan Inst. Chem. Eng. 2021, 122, 51–57. [Google Scholar] [CrossRef]
- Kumar Das, S.; Adediran, A.; Rodrigue Kaze, C.; Mohammed Mustakim, S.; Leklou, N. Production, Characteristics, and Utilization of Rice Husk Ash in Alkali Activated Materials: An Overview of Fresh and Hardened State Properties. Constr. Build. Mater. 2022, 345, 128341. [Google Scholar] [CrossRef]
- Carmona, V.B.; Oliveira, R.M.; Silva, W.T.L.; Mattoso, L.H.C.; Marconcini, J.M. Nanosilica from Rice Husk: Extraction and Characterization. Ind. Crops Prod. 2013, 43, 291–296. [Google Scholar] [CrossRef]
- Xu, W.; Wei, J.; Chen, J.; Zhang, B.; Xu, P.; Ren, J.; Yu, Q. Comparative Study of Water-Leaching and Acid-Leaching Pretreatment on the Thermal Stability and Reactivity of Biomass Silica for Viability as a Pozzolanic Additive in Cement. Materials 2018, 11, 1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fapohunda, C.; Akinbile, B.; Shittu, A. Structure and Properties of Mortar and Concrete with Rice Husk Ash as Partial Replacement of Ordinary Portland Cement—A Review. Int. J. Sustain. Built Environ. 2017, 6, 675–692. [Google Scholar] [CrossRef]
- Tharani, D.; Ananthasubramanian, M. Influence of Pre-Treatment Processes on the Purity and Characteristics of Silica Extracted from Rice Husk. Biomass Convers. Biorefin. 2023, in press. [Google Scholar] [CrossRef]
- Jamil, N.H.; Abdullah, S.A.; Zarib, N.S.M. Extraction Of Silica From Rice Husk Via Acid Leaching Treatment. In Technology & Society: A Multidisciplinary Pathway for Sustainable Development; Qureshi, M.I., Ed.; European Proceedings of Social and Behavioural Sciences: London, UK, 2019; pp. 175–183. [Google Scholar] [CrossRef]
- Kwan, W.H.; Wong, Y.S. Acid Leached Rice Husk Ash (ARHA) in Concrete: A Review. Mater. Sci. Energy Technol. 2020, 3, 501–507. [Google Scholar] [CrossRef]
- Ali, M.; Ul Haq, E.; Abdul Karim, M.R.; Ahmed, S.; Ibrahim, A.; Ahmad, W.; Baig, W.M. Effect of Leaching with 5-6 N H2SO4 on Thermal Kinetics of Rice Husk during Pure Silica Recovery. J. Adv. Res. 2016, 7, 47–51. [Google Scholar] [CrossRef]
- Aslam, U.; Ramzan, N.; Iqbal, T.; Kazmi, M.; Ikhlaq, A. Effect of Demineralization on the Physiochemical Structure and Thermal Degradation of Acid Treated Indigenous Rice Husk. Pol. J. Chem. Technol. 2016, 18, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Ma, Q.; Lin, Q.; Chang, J.; Ma, H. A Novel Approach to Extract SiO2 from Fly Ash and Its Considerable Adsorption Properties. Mater. Des. 2017, 116, 666–675. [Google Scholar] [CrossRef]
- Faizul, C.P.; Abdullah, C.; Fazlul, B. Extraction of Silica from Palm Ash Using Citric Acid Leaching Treatment: Preliminary Result. Adv. Mater. Res. 2013, 795, 7091–7706. [Google Scholar] [CrossRef]
- Montoneri, E.; Nisticò, R.; Francavilla, M. Demineralisation of Municipal Biowaste Hydrolysates. ChemistrySelect 2019, 4, 7551–7554. [Google Scholar] [CrossRef]
- Gautam, N.; Athira Merlin Rose, K.V.; Chaurasia, A. Study on Chemical Kinetics and Characterization of Nanosilica from Rice Husk and Rice Straw in the Fixed-Bed Pyrolysis Process. Biomass Convers. Biorefin. 2022, 12, 1435–1448. [Google Scholar] [CrossRef]
- Pratap, V.; Bombaywala, S.; Mandpe, A.; Khan, S.U. Solid Waste Treatment: Technological Advancements and Challenges. In Soft Computing Techniques in Solid Waste and Wastewater Management; Karri, R.R., Ravindran, G., Dehghani, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–231. [Google Scholar] [CrossRef]
- Gonçalves, M.R.F.; Bergmann, C.P. Thermal Insulators Made with Rice Husk Ashes: Production and Correlation between Properties and Microstructure. Constr. Build. Mater. 2007, 21, 2059–2065. [Google Scholar] [CrossRef]
- Akhayere, E.; Kavaz, D.; Vaseashta, A. Synthesizing Nano Silica Nanoparticles from Barley Grain Waste: Effect of Temperature on Mechanical Properties. Pol. J. Environ. Stud. 2019, 28, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Siddika, A.; Al Mamun, M.A.; Alyousef, R.; Mohammadhosseini, H. State-of-the-Art-Review on Rice Husk Ash: A Supplementary Cementitious Material in Concrete. J. King Saud Univ. Eng. Sci. 2021, 33, 294–307. [Google Scholar] [CrossRef]
- Şimşek, Y.E. Preparation and Characterization of High Purity Silica Obtained from Rice Husks. Acta Phys. Pol. A 2017, 132, 1002–1005. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, V.; Yadav, M.K.; Mohanta, K.; Majhi, M.R. A Comparative Study on Physio-Mechanical Properties of Silica Compacts Fabricated Using Rice Husk Ash Derived Amorphous and Crystalline Silica. Ceram. Int. 2022, 48, 35750–35758. [Google Scholar] [CrossRef]
- Genieva, S.; Turmanova, S.; Dimitrov, A.; Petkov, P.; Vlaev, L. Thermal Degradation of Rice Husks on a Pilot Plant. J. Therm. Anal. Calorim. 2012, 110, 111–118. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-Oil Production from Fast Pyrolysis of Rice Husk in a Commercial-Scale Plant with a Downdraft Circulating Fluidized Bed Reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Azat, S.; Korobeinyk, A.V.; Moustakas, K.; Inglezakis, V.J. Sustainable Production of Pure Silica from Rice Husk Waste in Kazakhstan. J. Clean. Prod. 2019, 217, 352–359. [Google Scholar] [CrossRef]
- Sarangi, M.; Nayak, P.; Tiwari, T.N. Effect of Temperature on Nano-Crystalline Silica and Carbon Composites Obtained from Rice-Husk Ash. Compos. B Eng. 2011, 42, 1994–1998. [Google Scholar] [CrossRef]
- Park, J.Y.; Mun, W.; Chun, J.; Sang, B.I.; Mitchell, R.J.; Lee, J.H. Alkali Extraction to Detoxify Rice Husk-Derived Silica and Increase Its Biocompatibility. ACS Sustain. Chem. Eng. 2022, 10, 7811–7817. [Google Scholar] [CrossRef]
- Wahab, R.A.A.; Zaid, M.H.M.; Ab Aziz, S.H.; Matori, K.A.; Fen, Y.W.; Yaakob, Y. Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source. Materials 2020, 13, 5413. [Google Scholar] [CrossRef] [PubMed]
- Rozainee, M.; Ngo, S.P.; Salema, A.A.; Tan, K.G. Fluidized Bed Combustion of Rice Husk to Produce Amorphous Siliceous Ash. Energy Sustain. Dev. 2008, 12, 33–42. [Google Scholar] [CrossRef]
- Goodman, B.A. Utilization of Waste Straw and Husks from Rice Production: A Review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- European Environment Agency Definition of Industrial Waste. Available online: http://www.eionet.europa.eu/gemet/aliss_scripts/concept/4273 (accessed on 31 May 2023).
- Hazardous Waste. Available online: https://eur-lex.europa.eu/EN/legal-content/glossary/hazardous-waste.html (accessed on 31 May 2023).
- Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics#Total_waste_generation (accessed on 31 May 2023).
- Vallero, D.A. Hazardous Wastes. In Waste: A Handbook for Management, 2nd ed.; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: London, UK, 2019; pp. 585–630. [Google Scholar] [CrossRef]
- Woszuk, A.; Panek, R.; Madej, J.; Zofka, A.; Franus, W. Mesoporous Silica Material MCM-41: Novel Additive for Warm Mix Asphalts. Constr. Build. Mater. 2018, 183, 270–274. [Google Scholar] [CrossRef]
- Panek, R.; Wdowin, M.; Franus, W.; Czarna, D.; Stevens, L.A.; Deng, H.; Liu, J.; Sun, C.; Liu, H.; Snape, C.E. Fly Ash-Derived MCM-41 as a Low-Cost Silica Support for Polyethyleneimine in Post-Combustion CO2 Capture. J. CO2 Util. 2017, 22, 81–90. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef] [Green Version]
- Wongkeo, W.; Thongsanitgarn, P.; Chaipanich, A. Compressive Strength and Drying Shrinkage of Fly Ash-Bottom Ash-Silica Fume Multi-Blended Cement Mortars. Mater. Des. 2012, 36, 655–662. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, H.; Li, P.; Li, Y.; Yan, B. Preparation of CaMgAl-LDHs and Mesoporous Silica Sorbents Derived from Blast Furnace Slag for CO2 Capture. RSC Adv. 2019, 9, 6054–6063. [Google Scholar] [CrossRef]
- Amdeha, E.; Mohamed, R.S.; Dhmees, A.S. Sonochemical Assisted Preparation of ZnS–ZnO/MCM-41 Based on Blast Furnace Slag and Electric Arc Furnace Dust for Cr (VI) Photoreduction. Ceram. Int. 2021, 47, 23014–23027. [Google Scholar] [CrossRef]
- Lin, L.Y.; Bai, H. Efficient Method for Recycling Silica Materials from Waste Powder of the Photonic Industry. Environ. Sci. Technol. 2013, 47, 4636–4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.Y.; Kuo, J.T.; Bai, H. Silica Materials Recovered from Photonic Industrial Waste Powder: Its Extraction, Modification, Characterization and Application. J. Hazard. Mater. 2011, 192, 255–262. [Google Scholar] [CrossRef]
- Bown, H.E.; Fuentes, J.P.; Martínez, A.M. Assessing Water Use and Soil Water Balance of Planted Native Tree Species under Strong Water Limitations in Northern Chile. New For. 2018, 49, 871–892. [Google Scholar] [CrossRef]
- Islam, K.; Vilaysouk, X.; Murakami, S. Integrating Remote Sensing and Life Cycle Assessment to Quantify the Environmental Impacts of Copper-Silver-Gold Mining: A Case Study from Laos. Resour. Conserv. Recycl. 2020, 154, 104630. [Google Scholar] [CrossRef]
- Covre, W.P.; Ramos, S.J.; Pereira, W.V.d.S.; de Souza, E.S.; Martins, G.C.; Teixeira, O.M.M.; do Amarante, C.B.; Dias, Y.N.; Fernandes, A.R. Impact of Copper Mining Wastes in the Amazon: Properties and Risks to Environment and Human Health. J. Hazard. Mater. 2022, 421, 126688. [Google Scholar] [CrossRef] [PubMed]
- Gleekia, A.M.G.D.; Pradhan, D.S.; Sahu, H.B. Impact of Iron Ore Mining on Water Quality and the Environment in Liberia. In Proceeding of the 6th Asian Mining Congress, Bhubaneswar, India, 23–27 February 2016. [Google Scholar]
- Wilson, S.A.; Wilson, C.O.; Moise, I.K. Livelihood Impacts of Iron Ore Mining-Induced Land Change in Sierra Leone: A Time Series Analysis. Appl. Geogr. 2022, 144, 102713. [Google Scholar] [CrossRef]
- Schueler, V.; Kuemmerle, T.; Schröder, H. Impacts of Surface Gold Mining on Land Use Systems in Western Ghana. AMBIO 2011, 40, 528–539. [Google Scholar] [CrossRef] [Green Version]
- Mardonova, M.; Han, Y.S. Environmental, Hydrological, and Social Impacts of Coal and Nonmetal Minerals Mining Operations. J. Environ. Manag. 2013, 332, 117387. [Google Scholar] [CrossRef]
- Fontes, W.C.; Franco de Carvalho, J.M.; Andrade, L.C.R.; Segadães, A.M.; Peixoto, R.A.F. Assessment of the Use Potential of Iron Ore Tailings in the Manufacture of Ceramic Tiles: From Tailings-Dams to “Brown Porcelain”. Constr. Build. Mater. 2019, 206, 111–121. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative Methodology for Comprehensive Utilization of Iron Ore Tailings. Part 1. The Recovery of Iron from Iron Ore Tailings Using Magnetic Separation after Magnetizing Roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Yang, T.; Feng, J.; Yang, H. Synthesis of Mesoporous Silica MCM-41 Using Sodium Silicate Derived from Copper Ore Tailings with an Alkaline Molted-Salt Method. J. Ind. Eng. Chem. 2015, 29, 338–343. [Google Scholar] [CrossRef]
- Yang, G.; Deng, Y.; Wang, J. Non-Hydrothermal Synthesis and Characterization of MCM-41 Mesoporous Materials from Iron Ore Tailing. Ceram. Int. 2014, 40, 7401–7406. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.; Wang, J.; Tan, Q.; Fu, L. Utilization of Iron Tailings to Prepare High-Surface Area Mesoporous Silica Materials. Sci. Total Environ. 2020, 736, 139483. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xue, X.; Huang, D. Synthesis of Mesoporous Silica Materials (MCM-41) from Iron Ore Tailings. Mater. Res. Bull. 2009, 44, 2112–2115. [Google Scholar] [CrossRef]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Mavumengwana, V. Gold Mine Tailings: A Potential Source of Silica Sand for Glass Making. Minerals 2020, 10, 448. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, K.; Bai, J.; Fang, B.; Wang, F. Cost-Effective Preparation of Gold Tailing-Based Aerogels for Efficient Adsorption of Copper Ions from Wastewater. Water 2023, 15, 669. [Google Scholar] [CrossRef]
- Kalisz, S.; Kibort, K.; Mioduska, J.; Lieder, M.; Małachowska, A. Waste Management in the Mining Industry of Metals Ores, Coal, Oil and Natural Gas—A Review. J. Environ. Manag. 2022, 304, 114239. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A Review of Circular Economy Strategies for Mine Tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Wong-Pinto, L.; Menzies, A.; Ordóñez, J.I. Bionanomining: Biotechnological Synthesis of Metal Nanoparticles from Mining Waste—Opportunity for Sustainable Management of Mining Environmental Liabilities. Appl. Microbiol. Biotechnol. 2020, 104, 1859–1869. [Google Scholar] [CrossRef]
- Ally, A.N.; Blanche, M.M.; Nana, U.J.P.; Grâce, M.M.; François, N.; Pettang, C. Recovery of Mining Wastes in Building Materials: A Review. Open J. Civil Eng. 2021, 11, 379–397. [Google Scholar] [CrossRef]
- Ubaldini, S.; Guglietta, D.; Vegliò, F.; Giuliano, V. Valorization of Mining Waste by Application of Innovative Thiosulphate Leaching for Gold Recovery. Metals 2019, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Li, S.; Zhang, C.; Bai, J.; Guo, Z. Application of Superconducting High Gradient Magnetic Separation Technology on Silica Extraction from Iron Ore Beneficiation Tailings. Miner. Process. Extr. Metall. Rev. 2018, 39, 44–49. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhao, X.; Pan, X.; Guo, P. Separation and Purification of High-Purity Quartz from High-Silicon Iron Ore Tailing: An Innovative Strategy for Comprehensive Utilization of Tailings Resources. Process Saf. Environ. Prot. 2023, 169, 142–148. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Bindar, Y. Operating Variables on Production of High Purity Biosilica from Rice Hull Ash by Extraction Process. J. Eng. Technol. Sci. 2022, 54, 220304. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Zemnukhova, L.A.; Kovshun, A.A.; Kovekhova, A.V. Processing Methods of Alkaline Hydrolysate from Rice Husk. Rice Sci. 2017, 24, 235–240. [Google Scholar] [CrossRef]
- Haq, I.U.; Akhtar, K.; Malik, A. Effect of Experimental Variables on the Extraction of Silica from the Rice Husk Ash. J. Chem. Soc. Pak. 2014, 36, 382. [Google Scholar]
- Alam, Q.; Hendrix, Y.; Thijs, L.; Lazaro, A.; Schollbach, K.; Brouwers, H.J.H. Novel Low Temperature Synthesis of Sodium Silicate and Ordered Mesoporous Silica from Incineration Bottom Ash. J. Clean. Prod. 2019, 211, 874–883. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; He, H.; Sun, Z.; Li, A. Silica Extraction from Bauxite Reaction Residue and Synthesis Water Glass. Green Process. Synth. 2021, 10, 268–283. [Google Scholar] [CrossRef]
- Jumari, A.; Yudha, C.S.; Widiyandari, H.; Lestari, A.P.; Rosada, R.A.; Santosa, S.P.; Purwanto, A. SiO2/C Composite as a High Capacity Anode Material of LiNi0.8Co0.15Al0.05O2 Battery Derived from Coal Combustion Fly Ash. Appl. Sci. 2020, 10, 8428. [Google Scholar] [CrossRef]
- Nisticò, R.; Scalarone, D.; Magnacca, G. Preparation and Physico-Chemical Characterization of Large-Mesopore Silica Thin Films Templated by Block Copolymers for Membrane Technology. Microporous Mesoporous Mater. 2014, 190, 208–214. [Google Scholar] [CrossRef]
- Nisticò, R.; Scalarone, D.; Magnacca, G. Sol-Gel Chemistry, Templating and Spin-Coating Deposition: A Combined Approach to Control in a Simple Way the Porosity of Inorganic Thin Films/Coatings. Microporous Mesoporous Mater. 2017, 248, 18–29. [Google Scholar] [CrossRef]
- Toure, A.O.; Sambe, F.M.; Koita, D.; Diop, C.M.G.; Sock, O. Processes for Working-up an Aqueous Fluosilicic Acid Solution. S. Afr. J. Sci. 2012, 108, 108–112. [Google Scholar] [CrossRef]
- Hexafluorosilicic Acid Safety Card. Available online: http://niosh.dnacih.com/nioshdbs/ipcsneng/neng1233.htm (accessed on 31 May 2023).
- Sarawade, P.B.; Kim, J.K.; Hilonga, A.; Kim, H.T. Recovery of High Surface Area Mesoporous Silica from Waste Hexafluorosilicic Acid (H2SiF6) of Fertilizer Industry. J. Hazard. Mater. 2010, 173, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Vu, C.M.; Choi, H.J.; Kien, B.X. Nanosilica Extracted from Hexafluorosilicic Acid of Waste Fertilizer as Reinforcement Material for Natural Rubber: Preparation and Mechanical Characteristics. Materials 2019, 12, 2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, F.; Wang, X.; Liu, T.; Xiao, L.; Yuan, M.; Fan, Y. Synthesis of ZSM-5 with the Silica Source from Industrial Hexafluorosilicic Acid as Transalkylation Catalyst. Chin. J. Chem. Eng. 2017, 25, 1303–1313. [Google Scholar] [CrossRef]
- Dreveton, A. Economic Aspects of Utilizing Fluosilicic Acid as Raw Material for the Manufacture of Hydrofluoric Acid and Aluminium Fluoride. Procedia Eng. 2014, 83, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Ayarza, N.; Góngora, J.M.G.; Alonso, R.M.; Jiménez, R.M. Determination of Anionic Impurities in Hexafluorosilicic Acid by Capillary Zone Electrophoresis. Anal. Methods 2012, 4, 3157–3162. [Google Scholar] [CrossRef]
- Yu, H.S.; Rhee, K.-I.; Lee, C.K.; Yang, D.-H. Two-Step Ammoniation of by-Product Fluosilicic Acid to Produce High Quality Amorphous Silica. Korean J. Chem. Eng. 2000, 17, 401–408. [Google Scholar] [CrossRef]
- Van Tuan, N.; Ye, G.; van Breugel, K.; Fraaij, A.; Dai Bui, D. The study of using rice husk ash to produce ultra high performance concrete. Construct. Build. Mater. 2011, 25, 2030–2035. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Satyanarayana, K.; Pramada, P.; Raghavan, P.; Gupta, T. Processing, properties and applications of reactive silica from rice husk—An overview. J. Mater. Sci. 2003, 38, 3159–3168. [Google Scholar] [CrossRef]
- Sahoo, S.; Parhi, P.; Chandra Panda, B. Durability properties of concrete with silica fume and rice husk ash. Clean. Eng. Technol. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Arayapranee, W.; Naranong, N.; Rempel, G. Application of rice husk ash as fillers in the natural rubber industry. J. Appl. Polym. Sci. 2005, 98, 34–41. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Rakdee, C.; Thanmathorn, P. Use of rice husk ash as filler in natural rubber vulcanizates: In comparison with other commercial fillers. J. Appl. Polym. Sci. 2002, 83, 2485–2493. [Google Scholar] [CrossRef]
- Choophun, N.; Chaiammart, N.; Sukthavon, K.; Veranitisagul, C.; Laobuthee, A.; Watthanaphanit, A.; Panomsuwan, G. Natural Rubber Composites Reinforced with Green Silica from Rice Husk: Effect of Filler Loading on Mechanical Properties. J. Comp. Sci. 2022, 6, 369. [Google Scholar] [CrossRef]
- Lavagna, L.; Nisticò, R. An Insight into the Chemistry of Cement—A Review. Appl. Sci. 2023, 13, 203. [Google Scholar] [CrossRef]
- Luo, T.; Hua, C.; Liu, F.; Sun, Q.; Yi, Y.; Pan, X. Effect of adding solid waste silica fume as a cement paste replacement on the properties of fresh and hardened concrete. Case Stud. Constr. Mater. 2022, 16, e01048. [Google Scholar] [CrossRef]
- Lolage, M.; Parida, P.; Chaskar, M.; Gupta, A.; Rautaray, D. Green Silica: Industrially scalable & sustainable approach towards achieving improved “nano filler-Elastomer” interaction and reinforcement in tire tread compounds. Sustain. Mater. Technol. 2020, 26, e00232. [Google Scholar] [CrossRef]
- Jomin, T.; Renuka, P. The Road to Sustainable Tire Materials: Current State-of-the-Art and Future Prospectives. Environ. Sci. Technol. 2023, 57, 2209–2216. [Google Scholar] [CrossRef]
- Goodyear.eu. Goodyear Sets Goal to Double Use of Rice Husk Ash Silica by 2021. Available online: https://news.goodyear.eu/goodyear-sets-goal-to-double-use-of-rice-husk-ash-silica-by-2021/ (accessed on 27 July 2023).
- Tyrepress.com. Pirelli Researching the Use of Rice Husk Ash in Tyre Compounds. Available online: https://www.tyrepress.com/2009/04/pirelli-researching-the-use-of-rice-husk-ash-in-tyre-compounds/ (accessed on 27 July 2023).
- Wu, L.; Li, Y.; Fu, Z.; Su, B.L. Hierarchically Structured Porous Materials: Synthesis Strategies and Applications in Energy Storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-Ouda, A.; Bak-Sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Parvanian, A.M.; Sadeghi, N.; Rafiee, A.; Shearer, C.J.; Jafarian, M. Application of Porous Materials for CO2 Reutilization: A Review. Energies 2022, 15, 63. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A Critical Review on VOCs Adsorption by Different Porous Materials: Species, Mechanisms and Modification Methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Thambiliyagodage, C. Porous Carbon Materials in Biomedical Applications. Biomed. J. Sci. Tech. Res. 2019, 22, 16905–16907. [Google Scholar] [CrossRef]
- Clyne, T.W.; Golosnoy, I.O.; Tan, J.C.; Markaki, A.E. Porous Materials for Thermal Management under Extreme Conditions. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous Materials for Hydrogen Storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Chmielarz, L.; Rutkowska, M.; Kowalczyk, A. Advances in Functionalization of Inorganic Porous Materials for Environmental Catalysis. Adv. Inorg. Chem. 2018, 72, 323–383. [Google Scholar] [CrossRef]
- Canossa, S.; Wuttke, S. Functionalization Chemistry of Porous Materials. Adv. Funct. Mater. 2020, 30, 2003875. [Google Scholar] [CrossRef]
- Wang, S. Ordered Mesoporous Materials for Drug Delivery. Microporous Mesoporous Materials 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Perovic, M.; Qin, Q.; Oschatz, M. From Molecular Precursors to Nanoparticles—Tailoring the Adsorption Properties of Porous Carbon Materials by Controlled Chemical Functionalization. Adv. Funct. Mater. 2020, 30, 1908371. [Google Scholar] [CrossRef]
- Cui, Y.; Lian, X.; Xu, L.; Chen, M.; Yang, B.; Wu, C.E.; Li, W.; Huang, B.; Hu, X. Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials 2019, 12, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Yu, X.Y.; Lou, X.W. The Design and Synthesis of Hollow Micro-/Nanostructures: Present and Future Trends. Adv. Mater. 2018, 30, 1800939. [Google Scholar] [CrossRef] [PubMed]
- Schwanke, A.J.; Gomes, J.F.; Bernardo-Gusmão, K.; Pergher, S. Combining Soft- And Hard-Templating Approaches in MWW-Type Zeolites. Molecules 2020, 25, 3335. [Google Scholar] [CrossRef] [PubMed]
- Stucki, M.; Loepfe, M.; Stark, W.J. Porous Polymer Membranes by Hard Templating—A Review. Adv. Eng. Mater. 2018, 20, 1700611. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Song, L.; Li, H.; Hou, F. Preparation and Characterization of Encapsulation of Europium Complex into Meso-Structured Silica Monoliths Using PEG as the Template. Microporous Mesoporous Mater. 2009, 122, 7–12. [Google Scholar] [CrossRef]
- Irfan Khan, M.; Azizli, K.; Sufian, S.; Man, Z.; Khan, A.S. Simultaneous Preparation of Nano Silica and Iron Oxide from Palm Oil Fuel Ash and Thermokinetics of Template Removal. RSC Adv. 2015, 5, 20788–20799. [Google Scholar] [CrossRef]
- Rahman, N.A.; Widhiana, I.; Juliastuti, S.R.; Setyawan, H. Synthesis of Mesoporous Silica with Controlled Pore Structure from Bagasse Ash as a Silica Source. Colloids Surf. A Physicochem. Eng. Asp. 2015, 476, 1–7. [Google Scholar] [CrossRef]
- Andrade, G.F.; Soares, D.C.F.; Dos Santos, R.G.; Sousa, E.M.B. Mesoporous Silica SBA-16 Nanoparticles: Synthesis, Physicochemical Characterization, Release Profile, and in Vitro Cytocompatibility Studies. Microporous Mesoporous Mater. 2013, 168, 102–110. [Google Scholar] [CrossRef]
- Kalu, O.I.; Subramanian, B.; MacLean, B.J.; Saha, G.C. A Novel Approach to the Sol–Gel Synthesis of Titanium Dioxide-Coated SBA-16 Type Silica Mesoporous Microspheres for Water Purification. Materialia 2019, 5, 100237. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized Mesoporous SBA-15 Silica: Recent Trends and Catalytic Applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Hafezian, S.M.; Azizi, S.N.; Biparva, P.; Bekhradnia, A. High-Efficiency Purification of Sulforaphane from the Broccoli Extract by Nanostructured SBA-15 Silica Using Solid-Phase Extraction Method. J. Chromatogr. B 2019, 1108, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Avetta, P.; Calza, P.; Fabbri, D.; Magnacca, G.; Scalarone, D. Selective Porous Gates Made from Colloidal Silica Nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2105–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisticò, R.; Magnacca, G.; Jadhav, S.A.; Scalarone, D. Polystyrene-Block-Poly(Ethylene Oxide) Copolymers as Templates for Stacked, Spherical Large-Mesopore Silica Coatings: Dependence of Silica Pore Size on the PS/PEO Ratio. Beilstein J. Nanotechnol. 2016, 7, 1454–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 Capture with Pore-Expanded MCM-41 Silica Modified with Amino Groups by Double Functionalization. Microporous Mesoporous Materials 2015, 209, 165–171. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.H.; Cho, E.B. Recent Trends in Morphology-Controlled Synthesis and Application of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef]
- Park, S.B.; Joo, Y.H.; Kim, H.; Ryu, W.; Park, Y.I. Biodegradation-Tunable Mesoporous Silica Nanorods for Controlled Drug Delivery. Mater. Sci. Eng. C 2015, 50, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, Z.; Zhang, L.; Li, Y.; Yang, J.; Shen, J.; Wang, J.; Niu, Y.; Xiao, Z.; Chen, L.; et al. Preparation of Hollow Mesoporous Silica Nanorods for Encapsulating and Slowly Releasing Eugenol. Chin. Chem. Lett. 2020, 31, 3135–3138. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of Large Pore MCM-41 Molecular Sieves to Improve Pore Size Analysis Using Nitrogen Adsorption Measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Şahin, R.Z.Y. Understanding the Effect of Calcination Process on the Mesoporous MCM-41 Material Morphology. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2021, 4, 27–34. [Google Scholar]
- Palencia-Ruiz, S.; Sachse, A.; Amar, F.; Gucuyener, C.; Bats, N.; Batalha, N.; Pinard, L. Understanding the Mechanism of Large-Scale Template Elimination during Calcination of Mcm-41. Microporous Mesoporous Mater. 2022, 338, 111981. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- de Freitas, F.A.; Keils, D.; Lachter, E.R.; Maia, C.E.B.; Pais da Silva, M.I.; Veiga Nascimento, R.S. Synthesis and Evaluation of the Potential of Nonionic Surfactants/Mesoporous Silica Systems as Nanocarriers for Surfactant Controlled Release in Enhanced Oil Recovery. Fuel 2019, 241, 1184–1194. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Dudzinska, P.; Nowicki, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Novel Mesoporous Organosilicas with Task Ionic Liquids: Properties and High Adsorption Performance for Pb(II). Molecules 2022, 27, 1405. [Google Scholar] [CrossRef] [PubMed]
- Beagan, A.; Alotaibi, K.; Almakhlafi, M.; Algarabli, W.; Alajmi, N.; Alanazi, M.; Alwaalah, H.; Alharbi, F.; Alshammari, R.; Alswieleh, A. Amine and Sulfonic Acid Functionalized Mesoporous Silica as an Effective Adsorbent for Removal of Methylene Blue from Contaminated Water. J. King Saud Univ. Sci. 2022, 34, 101762. [Google Scholar] [CrossRef]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A Novel Mesoporous Fe-Silica Aerogel Composite with Phenomenal Adsorption Capacity for Malachite Green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Elimbinzi, E.; Nyandoro, S.S.; Mubofu, E.B.; Manayil, J.C.; Lee, A.F.; Wilson, K. Valorization of Rice Husk Silica Waste: Organo-Amine Functionalized Castor Oil Templated Mesoporous Silicas for Biofuels Synthesis. Microporous Mesoporous Mater. 2020, 294, 109868. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Tsai, Y.C.; Lin, H.P.; Hsu, C.H. Rice Husk-Derived Hierarchical Micro/Mesoporous Carbon–Silica Nanocomposite as Superior Filler for Green Electronic Packaging Material. J. Chin. Chem. Soc. 2017, 64, 427–433. [Google Scholar] [CrossRef]
- Liou, T.H.; Tseng, Y.K.; Liu, S.M.; Lin, Y.T.; Wang, S.Y.; Liu, R.T. Green Synthesis of Mesoporous Graphene Oxide/Silica Nanocomposites from Rich Husk Ash: Characterization and Adsorption Performance. Environ. Technol. Innov. 2021, 22, 101424. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Zhu, J.; Bai, J.; Gao, L.; Liu, S.; Jiao, T. Facile Preparation of Self-Assembled Chitosan-Based Composite Hydrogels with Enhanced Adsorption Performances. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124860. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Li, Y.X.; Liu, Z. Novel Adsorption Materials Based on Graphene Oxide/Beta Zeolite Composite Materials and Their Adsorption Performance for Rhodamine B. J. Alloys Compd. 2017, 708, 255–263. [Google Scholar] [CrossRef]
- Wang, J.; Tsuzuki, T.; Tang, B.; Hou, X.; Sun, L.; Wang, X. Reduced Graphene Oxide/ZnO Composite: Reusable Adsorbent for Pollutant Management. ACS Appl. Mater. Interfaces 2012, 4, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics 2022, 10, 87. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, C.; Fang, H.; Zhu, W.; Shi, J.; Liu, G. Synthesis of Ordered Mesoporous Silica from Biomass Ash and Its Application in CO2 Adsorption. Environ. Res. 2023, 231, 116070. [Google Scholar] [CrossRef] [PubMed]
- Motawea, M.M.; Hussein, M.A.T.; Elsenety, M.M.; Ali, H.M.; Seaf El-Nasr, T.A.; Gomaa, H. Mesoporous Hierarchical ZrO2@rice Straw-Derived SiO2 Nanocomposite for Rapid Adsorption and Sunlight-Driven Photocatalytic Degradation of Methylene Blue. J. Photochem. Photobiol. A Chem. 2022, 426, 113758. [Google Scholar] [CrossRef]
- Gomaa, H.; Hussein, M.A.T.; Motawea, M.M.; Aboraia, A.M.; Cheira, M.F.; Alotaibi, M.T.; El-Bahy, S.M.; Ali, H.M. A Hybrid Mesoporous CuO@barley Straw-Derived SiO2 Nanocomposite for Adsorption and Photocatalytic Degradation of Methylene Blue from Real Wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128811. [Google Scholar] [CrossRef]
- Liou, T.H.; Liu, S.M.; Chen, G.W. Utilization of E-Wastes as a Sustainable Silica Source in Synthesis of Ordered Mesostructured Titania Nanocomposites with High Adsorption and Photoactivity. J. Environ. Chem. Eng. 2022, 10, 107283. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zhao, M. Review on Template Removal Techniques for Synthesis of Mesoporous Silica Materials. Energy Fuels 2022, 36, 2424–2446. [Google Scholar] [CrossRef]
- Abdul Razak, N.A.; Othman, N.H.; Mat Shayuti, M.S.; Jumahat, A.; Sapiai, N.; Lau, W.J. Agricultural and industrial waste-derived mesoporous silica nanoparticles: A review on chemical synthesis route. J. Environ. Chem. Eng. 2022, 10, 107322. [Google Scholar] [CrossRef]













| Agricultural Waste | SiO2 (wt.%) | K2O (wt.%) | Na2O (wt.%) | CaO (wt.%) | MgO (wt.%) | Al2O3 (wt.%) | Fe2O3 (wt.%) | ZnO (wt.%) | MnO2 (wt.%) | SO3 (wt.%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice husk ash | 89.61 | 2.53 | 0.16 | 1.52 | 0.56 | 0.36 | 0.90 | - | - | - | [87] |
| 79.63 | 3.83 | 7.04 | 2.36 | - | 1.86 | 1.04 | 0.09 | - | - | [84] | |
| 93.10 | 0.04 | 0.96 | 1.52 | 0.65 | 0.07 | - | - | - | 0.09 | [82] | |
| Sugar bagasse ash | 75.96 | 5.24 | - | 3.85 | 2.53 | 2.24 | 5.08 | - | - | - | [75] |
| Wheat straw | 88.09 | 1.84 | 0.36 | 2.22 | 0.99 | - | 0.48 | - | 0.02 | [77] | |
| 10.80 | 2.01 | - | 0.28 | 0.18 | - | 0.37 | - | - | - | [78] | |
| Corncob ash | 27.80 | 18.49 | - | 14.03 | 9.50 | 5.70 | 4.69 | - | - | - | [80] |
| 68.70 | 4.56 | 4.60 | 9.50 | 5.20 | - | 3.44 | - | 0.14 | 0.08 | [79] | |
| Bamboo leaf ash | 74.41 | - | 10.30 | 6.51 | 4.07 | 1.13 | 1.55 | - | 0.07 | 1.44 | [76] |
| Oxides | Composition of SiO2 Obtained from Barley Husk at Different Treatment Conditions | |||
|---|---|---|---|---|
| at 400 °C (%) | at 500 °C (%) | at 600 °C (%) | at 700 °C (%) | |
| SiO2 | 93.0 | 93.5 | 93.5 | 94.1 |
| CaO | 1.0 | 0.9 | 0.9 | 0.7 |
| MgO | 0.9 | 0.8 | 0.8 | 0.8 |
| K2O | 2.1 | 2.0 | 2.0 | 1.9 |
| Fe2O3 | 0.5 | 0.3 | 0.3 | 0.3 |
| P2O5 | 0.6 | 0.6 | 0.6 | 0.6 |
| Al2O3 | 0.7 | 0.7 | 0.7 | 0.7 |
| B2O3 | 1.2 | 1.2 | 1.2 | 0.9 |
| Mining Waste | SiO2 (wt.%) | K2O (wt.%) | Na2O (wt.%) | CaO (wt.%) | MgO (wt.%) | Al2O3 (wt.%) | Fe2O3 (wt.%) | ZnO (wt.%) | TiO2 (wt.%) | CuO (wt.%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper ore tailing | 68.23 | - | 1.43 | 5.27 | 1.44 | 5.01 | 10.16 | - | - | 0.12 | [133] |
| Iron ore tailing | 50.88 | - | . | 3.26 | 0.59 | 13.62 | 25.18 | - | 0.28 | - | [134] |
| 76.70 | 0.26 | 0.30 | 1.48 | 2.93 | 1.95 | 15.77 | 0.01 | 0.03 | 0.01 | [135] | |
| 82.26 | - | - | 0.57 | - | 0.80 | 14.37 | - | 0.02 | - | [136] | |
| Gold mine tailing | 89.03 | 1.53 | 0.17 | 0.21 | 0.26 | 4.43 | 1.42 | - | 0.44 | - | [137] |
| 57.70 | 3.74 | 3.02 | 5.97 | 3.77 | 17.20 | 6.33 | - | 0.93 | - | [138] |
| Mesoporous SiO2 NP Family | Acronym | Pore Symmetry | Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|---|
| M41S | MCM-41 | 2D hexagonal | 1.5–8 | >1.00 |
| MCM-48 | 3D cubic | 2–5 | >1.00 | |
| MCM-50 | Lamellar | 2–5 | >1.00 | |
| SBA | SBA-11 | 3D cubic | 2.1–3.6 | 0.68 |
| SBA-12 | 3D hexagonal | 3.1 | 0.83 | |
| SBA-15 | 2D hexagonal | 6.0 | 1.17 | |
| SBA-16 | Cubic | 5–15 | 0.91 | |
| KIT | KIT-5 | Cubic | 9.3 | 0.45 |
| COK | COK-12 | Hexagonal | 5.8 | 0.45 |
| FDU | FDU-12 | 3D cubic | 10–26 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montini, D.; Cara, C.; D’Arienzo, M.; Di Credico, B.; Mostoni, S.; Nisticò, R.; Pala, L.; Scotti, R. Recent Advances on Porous Siliceous Materials Derived from Waste. Materials 2023, 16, 5578. https://doi.org/10.3390/ma16165578
Montini D, Cara C, D’Arienzo M, Di Credico B, Mostoni S, Nisticò R, Pala L, Scotti R. Recent Advances on Porous Siliceous Materials Derived from Waste. Materials. 2023; 16(16):5578. https://doi.org/10.3390/ma16165578
Chicago/Turabian StyleMontini, Daniele, Claudio Cara, Massimiliano D’Arienzo, Barbara Di Credico, Silvia Mostoni, Roberto Nisticò, Luca Pala, and Roberto Scotti. 2023. "Recent Advances on Porous Siliceous Materials Derived from Waste" Materials 16, no. 16: 5578. https://doi.org/10.3390/ma16165578