Electrochemical Response of 3D-Printed Free-Standing Reduced Graphene Oxide Electrode for Sodium Ion Batteries Using a Three-Electrode Glass Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Three-Dimensional Printing of rGO Scaffold
2.2. Electrochemical Measurements
2.3. Microstructural Characterization
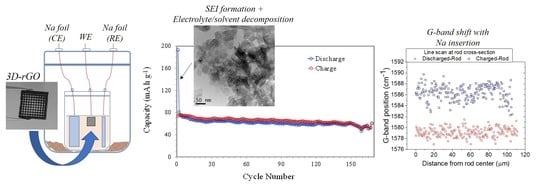
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushi, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar]
- Yusuf, M.; Kumar, M.; Khan, M.A.; Sillanpääillanpa, M.; Arafat, H. A review on exfoliation, characterization, environmental and energy applications of graphene and graphene-based composites. Adv. Colloid Interface Sci. 2019, 273, 102036. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Applications of ceramic/graphene composites and hybrids. Materials 2021, 14, 2071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, A.; Shen, C.; Liu, Q.; Cao, X.; Ma, Y.; Chen, L.; Lau, C.; Chen, T.C.; Wei, F.; et al. Red phosphorus nanodots on reduced graphene oxide as a flexible and ultra-fast anode for sodium-ion batteries. ACS Nano 2017, 11, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- El Haj Assad, M.; Khosravi, A.; Malekan, M.; Rosen, M.A.; Nazari, M.A. Chapter 14-Energy storage. In Design and Performance Optimization of Renewable Energy Systems; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Wasalathilake, K.C.; Li, H.; Xu, L.; Yan, C. Recent advances in graphene based materials as anode materials in sodium-ion batteries. J. Energy Chem. 2020, 42, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium-air and sodium-sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar]
- Zhu, J.; Duan, R.; Zhang, S.; Jiang, N.; Zhang, Y.; Zhu, J. The application of graphene in lithium ion battery electrode materials. Springer Plus 2014, 3, 585. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and graphene-based composites as Li-ion battery electrode materials and their application in full cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Y. Review of Graphene in cathode materials for lithium-ion batteries. Energy Fuels 2021, 35, 3572–3580. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From charge storage mechanism to performance: A roadmap toward high specific energy sodium-ion batteries through carbon anode optimization. Adv. Energy Mater. 2018, 8, 1703268. [Google Scholar] [CrossRef]
- Park, J.; Sharma, J.; Jafta, C.J.; He, L.; Meyer, H.M.; Li, J.; Keum, J.K.; Nguyen, N.A.; Polizos, G. Reduced graphene oxide aerogels with functionalization- mediated disordered stacking for sodium-ion batteries. Batteries 2022, 8, 12. [Google Scholar] [CrossRef]
- Luo, D.; Xu, J.; Guo, Q.; Fang, L.; Zhu, X.; Xia, Q.; Xia, H. Surface-dominated sodium storage towards high capacity and ultrastable anode material for sodium-ion batteries. Adv. Funct. Mater. 2018, 28, 1805371. [Google Scholar] [CrossRef]
- Luo, X.F.; Yang, C.H.; Peng, Y.Y.; Pu, N.W.; Ger, M.D.; Hsieh, C.T.; Chang, J.K. Graphene nanosheets, carbon nanotubes, graphite, and activated carbon as anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 10320–10326. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.; Dai, L. High-performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Mehmood, A.; Ha, H.Y.; Kim, J.; Chung, K.Y. Reduced graphene oxide as a stable and high-capacity cathode material for Na-ion batteries. Sci. Rep. 2017, 7, 40910. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Lim, G.J.H.; Koh, J.J.; Li, Y.; Ma, Y.; Ding, J.; Wang, J.; Hu, Z.; Wang, J.; Chen, W.; et al. Design and manufacture of 3D-printed batteries. Joule 2021, 5, 89–114. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Chi, L.; Liu, Y.; Zhang, Y.; Wang, K.; Wu, Z.S. 3D Printing Flexible Sodium-Ion Microbatteries with Ultrahigh Areal Capacity and Robust Rate Capability. Adv. Mater. 2022, 39, 2205569. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Hou, Z.; Ta, W.; Wang, W.; Zhao, X.; Zhang, Q.; Quan, Z. 3D printing of hierarchical graphene lattice for advanced Na metal anodes. ACS Appl. Energy Mater. 2019, 2, 3869–3877. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Li, W.; Tian, B.; Xu, T.; Kong, D.; Huang, S.; Liu, K.; Li, X.; Yang, H.Y.; et al. A simple and effective host for sodium metal anode: A 3D-printed high pyrrolic-N doped graphene microlattice aerogel. J. Mater. Chem. A 2022, 10, 16842–16852. [Google Scholar] [CrossRef]
- Yan, J.; Zhi, G.; Kong, D.; Wang, H.; Xu, T.; Zang, J.; Shen, W.; Xu, J.; Shi, Y.; Dai, S.; et al. 3D printed rGO/CNT microlattice aerogel for a dendrite-free sodium metal anode. J. Mater. Chem. A 2020, 8, 19843–19854. [Google Scholar] [CrossRef]
- Ding, J.; Shen, K.; Du, Z.; Li, B.; Yang, S. 3D-printed hierarchical porous frameworks for sodium storage. ACS Appl. Mater. Interfaces 2017, 9, 41871–41877. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, J.; Gao, X.; Wang, S.; Sun, Q.; Luo, J.; Zhao, C.; Zhao, Y.; Yang, X.; Wang, C.; et al. 3D printing of free-standing “o2Breathable” air electrodes for high-capacity and long-life Na-O2 batteries. Chem. Mater. 2020, 32, 3018–3027. [Google Scholar] [CrossRef]
- Wang, H.; Bai, W.; Kong, D.; Xu, T.; Zhang, Z.; Zang, J.; Wang, X.; Zhang, S.; Tian, Y.; Li, X.; et al. 3D printed Au/rGO microlattice host for dendrite-free sodium metal anode. Energy Storage Mater. 2023, 55, 631–641. [Google Scholar] [CrossRef]
- Hawes, G.F.; Rehman, S.; Rangom, Y.; Pope, M.A. Advanced manufacturing approaches for electrochemical energy storage devices devices. Int. Mater. Rev. 2022, 68, 323–364. [Google Scholar] [CrossRef]
- Moyano, J.J.; Gómez-Gómez, A.; Pérez-Coll, D.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Filament printing of graphene-based inks into self-supported 3D architectures. Carbon 2019, 151, 94–102. [Google Scholar] [CrossRef]
- Moyano, J.J.; Mosa, J.; Aparicio, M.; Pérez-Coll, D.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Strong and light cellular silicon carbonitride—Reduced graphene oxide material with enhanced electrical conductivity and capacitive response. Addit. Manuf. 2019, 30, 100849. [Google Scholar] [CrossRef]
- Sun, J.; Sadd, M.; Edenborg, P.; Grönbeck, H.; Thiesen, P.H.; Xia, Z.; Quintano, V.; Qiu, R.; Matic, A.; Palermo, V. Real-time imaging of Na+ reversible intercalation in “Janus” graphene stacks for battery applications. Sci. Adv. 2021, 7, eabf0812. [Google Scholar] [CrossRef]
- Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X. Reduced graphene oxide with superior cycling stability and rate capability for sodium storage. Carbon 2013, 57, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Roman-Manso, B.; Figueiredo, F.M.; Achiaga, B.; Barea, R.; Perez-Coll, D.; Morelos-Gomez, A.; Terrones, M.; Osendi, M.I.; Belmonte, M.; Miranzo, P. Electrically functional 3D-architectured graphene/SiC composites. Carbon 2016, 100, 318–328. [Google Scholar] [CrossRef]
- David, L.; Singh, G. Reduced graphene oxide paper electrode: Opposing effect of thermal annealing on Li and Na cyclability. J. Phys. Chem. C. 2014, 118, 28401–28408. [Google Scholar] [CrossRef]
- Xu, S.; Lu, Y.; Wang, H.; Abruña, H.D.; Archer, L.A. A rechargeable Na-CO2/O2 battery enabled by stable nanoparticle hybrid electrolytes. J. Mater. Chem. A 2014, 2, 17723–17729. [Google Scholar] [CrossRef]
- Yin, W.W.; Fu, Z.W. The potential of Na–Air batteries. ChemCatChem 2017, 9, 1545–1553. [Google Scholar] [CrossRef]
- Kwak, W.J.; Chen, Z.; Yoon, C.S.; Lee, J.K.; Amine, K.; Sun, Y.K. Nanoconfinement of low-conductivity products in rechargeable sodium-air batteries. Nano Energy 2015, 12, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lim, H.D.; Gwon, H.; Kang, K. Sodium-oxygen batteries with alkyl-carbonate and ether based electrolytes. Phys. Chem. Chem. Phys. 2013, 15, 3623–3629. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]
- De La Llave, E.; Borgel, V.; Zinigrad, E.; Chesneau, F.F.; Hartmann, P.; Sun, Y.K.; Aurbach, D. Study of the most relevant aspects related to hard carbons as anode materials for Na-ion batteries, compared with Li-ion systems. Isr. J. Chem. 2015, 55, 1260–1274. [Google Scholar] [CrossRef]
- Mosallanejad, B.; Malek, S.S.; Ershadi, M.; Daryakenari, A.A.; Cao, Q.; Boorboor Ajdari, F.; Ramakrishna, S. Cycling degradation and safety issues in sodium-ion batteries: Promises of electrolyte additives. J. Electroanal. Chem. 2021, 895, 115505. [Google Scholar] [CrossRef]
- Wan, J.; Shen, F.; Luo, W.; Zhou, L.; Dai, J.; Han, X.; Bao, W.; Xu, Y.; Panagiotopoulos, J.; Fan, X.; et al. In situ transmission electron microscopy observation of sodiation-desodiation in a long cycle, high-capacity reduced graphene oxide sodium-ion battery anode. Chem. Mater. 2016, 28, 6528–6535. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Helen, M.; Groß, A.; Fichtner, M.; Euchner, H. Insight into sodium insertion and the storage mechanism in hard carbon. ACS Energy Lett. 2018, 3, 2851–2857. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Weaving, J.S.; Lim, A.; Millichamp, J.; Neville, T.P.; Ledwoch, D.; Kendrick, E.; McMillan, P.F.; Shearing, P.R.; Howard, C.A.; Brett, D.J. Elucidating the Sodiation Mechanism in Hard Carbon by Operando Raman Spectroscopy. ACS Appl. Energy Mater. 2020, 3, 7474–7484. [Google Scholar] [CrossRef]
- Tang, B.; Guoxin, H.; Gao, H. Raman spectroscopic characterization of graphene. Appl. Spectrosc. Rev. 2010, 45, 369–407. [Google Scholar] [CrossRef]
- Won, K.; Lee, C.; Jung, J.; Kwon, S.; Gebredingle, Y.; Lim, J.G.; Kim, M.K.; Jeong, M.S.; Lee, C. Raman scattering measurement of suspended graphene under extreme strain induced by nanoindentation. Adv. Mater. 2022, 34, 2200946. [Google Scholar] [CrossRef]
- Tonnoir, H.; Huo, D.; Davoisne, C.; Celzard, A.; Fierro, V.; Saurel, D.; El Marssi, M.; Benyoussef, M.; Meunier, P.; Janot, R. Pyrolysis temperature dependence of sodium storage mechanism in non-graphitizing carbons. Carbon 2023, 208, 216–226. [Google Scholar] [CrossRef]
- Ray, A.; Saruhan, B. Application of ionic liquids for batteries and supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Zhang, F.; Liang, H.; Ming, F.; Alshareef, H.N.; Gao, Z. Partially Reduced Holey Graphene Oxide as High Performance Anode for Sodium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803215. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, C. Free-standing reduced graphene oxide/carbon nanotube paper for flexible sodium-ion battery applications. Molecules 2020, 25, 1014. [Google Scholar] [CrossRef] [Green Version]










| ρbulk (g · cm−3) | ρsolid (g · cm−3) | Pskeleton (%) | Pmacro (%) | Ptotal (%) |
|---|---|---|---|---|
| 0.13 | 0.38 | 83 | 62 | 94 |
| Sample * | D (cm−1) | G (cm−1) | ID/IG |
|---|---|---|---|
| 3D rGO Na/charged, in depth | 1350 ±1 | 1586 ±1 | 1.9 ±0.1 |
| 3D rGO Na/discharged, in depth | 1350 +/−1 | 1578 +/−1 | 1.7 +/−0.1 |
| 3D rGO original | 1355 ±1 | 1596 ±1 | 1.9 ±0.1 |
| Electrode Material | Excitation Wavelength (nm) | Δ G-Band Position (cm−1) by Insertion/De-Insertion | Reference |
|---|---|---|---|
| Hard carbon synthesized from coconut shell | 633 | 50 | [42] |
| Commercial hard carbon | ---- | 25 | [43] |
| Commercial plant-based hard carbon | 532 | 38 | [44] |
| Ground pyrolyzed carbon | 532 | 38 | [47] |
| 633 | 41 | ||
| 780 | 42 |
| Electrode Material | Design | Cell Test | Discharge Capacity (mAh g−1) | Cycle Life | Reference |
|---|---|---|---|---|---|
| rGO/carbon black/PVDF | Bulk, 100 µm thickness | Coin cell | 141 at 40mA g−1 | 1000 at 40 mA g−1 | [31] |
| rGO/carbon black/PVDF | Bulk, 30 µm thickness | Coin cell | 235 at 30 mA g−1 | 1000 at 30 mA g−1 | [18] |
| rGO/carbon black/methyl cellulose | Bulk | Coin cell | 603 at 0.05 A g−1 | 10000 at 5 A g−1 | [15] |
| Holey rGO/carbon black/PTFE | Bulk | Coin cell | 365 at 0.1 A g−1 | 3000 at 2 A g−1 | [49] |
| rGO/CNT paper | Bulk, 12 µm | Coin cell | 166 at 0.05 A g−1 | 300 at 200 mA g−1 | [50] |
| rGO | Aerogel | Coin cell | 250 at 0.05 C | 20 at 0.05 C | [14] |
| Cyclodextrin rGO | Aerogel | Coin cell | 500 at 0.05 C | 100 at 1 C | [14] |
| rGO/carbon black/PVDF | Foam | Coin cell | 800 at 0.1 A g−1 | 150 at 500 mA g−1 | [17] |
| Na@rGO | 3D, 250 µm thickness | Coin cell | 500 at 1 mA cm−2 | [21] | |
| rGO/Na3V2(PO4)3 | 3D-printed | Coin cell (full cell) | 95 at 0.1 A g−1 | 1000 at 100 mA g−1 | [22] |
| Na@rGO/CNT | 3D-printed | Coin cell | 640 at 8 mA cm−2 | [23] | |
| rGO/Na3V2(PO4)3 | 3D-printed | Coin cell | 1.26 mAh cm−2 (areal capacity) at 0.2 C | 900 at 1 C | [24] |
| rGO (Na-O2 battery) | 3D-printed | Swagelok cell | 500 at 0.5 A g−1 | 122 at 0.5 A g−1 | [25] |
| rGO | 3D-printed | Three-electrode cell | 80 at 10 mA g−1 | 150 at 10 mA g−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, C.; Osendi, M.I.; Moyano, J.J.; Mosa, J.; Aparicio, M. Electrochemical Response of 3D-Printed Free-Standing Reduced Graphene Oxide Electrode for Sodium Ion Batteries Using a Three-Electrode Glass Cell. Materials 2023, 16, 5386. https://doi.org/10.3390/ma16155386
Ramírez C, Osendi MI, Moyano JJ, Mosa J, Aparicio M. Electrochemical Response of 3D-Printed Free-Standing Reduced Graphene Oxide Electrode for Sodium Ion Batteries Using a Three-Electrode Glass Cell. Materials. 2023; 16(15):5386. https://doi.org/10.3390/ma16155386
Chicago/Turabian StyleRamírez, Cristina, María Isabel Osendi, Juan José Moyano, Jadra Mosa, and Mario Aparicio. 2023. "Electrochemical Response of 3D-Printed Free-Standing Reduced Graphene Oxide Electrode for Sodium Ion Batteries Using a Three-Electrode Glass Cell" Materials 16, no. 15: 5386. https://doi.org/10.3390/ma16155386