High Entropy Borides Synthesized by the Thermal Reduction of Metal Oxides in a Microwave Plasma
Abstract
:1. Introduction
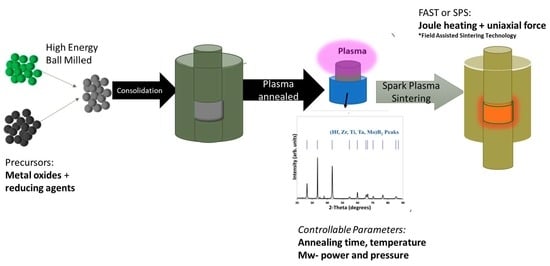
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Oxidation Resistance
3.3. HEB Microstructure
3.4. Hardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Jiang, Z.-B.; Sun, S.-K.; Guo, W.-M.; Chen, Q.-S.; Qiu, J.-X.; Plucknett, K.; Lin, H.-T. Microstructure and mechanical properties of high-entropy borides derived from boro/carbothermal reduction. J. Eur. Ceram. Soc. 2019, 39, 3920–3924. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.-K.; Guo, W.-M.; Xu, L.; Zhang, W.; Lin, H.-T. Optimal preparation of high-entropy boride-silicon carbide ceramics. J. Adv. Ceram. 2021, 10, 173–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, L.; Chai, Y.-F.; Guo, W.-M.; Zhang, T.-Q.; Xu, L.; Cong, J.-L.; Lin, H.-T.; Wei, L.-Y.; Ma, W.-M. Influence of Cr content on sintering, textured structure, and properties of (Hf, Zr, Ta, Cr, Ti) B2 high-entropy boride ceramics. Ceram. Int. 2023, 49, 16029–16037. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, Y.; Gao, Y.; Bi, J.; Li, Y.; Liu, C.; Gao, J.; Wang, W.; Qian, Z. First-principles prediction, fabrication and characterization of (Hf0.2Nb0.2Ta0.2Ti0.2Zr0.2)B2 high-entropy borides. Ceram. Int. 2022, 48, 17234–17245. [Google Scholar] [CrossRef]
- Zou, Q.; Gu, H.; Li, Y.; Li, Z.; Liang, P.; Luo, Y. Characterization and analysis of high-entropy boride ceramics sintered at low temperature. J. Am. Ceram. Soc. 2023, 106, 2764–2772. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Tian, H.; Liu, J.; Li, C.; Dong, H.; Chen, J.; Song, M.; Chen, B.; Sheng, H. Ultra-incompressible high-entropy diborides. J. Phys. Chem. Lett. 2021, 12, 3106–3113. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Zeng, Y.; Zhang, H.; Li, Z. Low-temperature high-efficiency preparation of TiB2 micro-platelets via boro/carbothermal reduction in microwave heated molten salt. Materials 2019, 12, 2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Gong, Y.; Song, S.; Yu, G.; Ma, S. Synthesis of high-entropy boride powders via boro/carbothermal reduction method. J. Asian Ceram. Soc. 2021, 9, 1275–1281. [Google Scholar] [CrossRef]
- Jermolovicius, L.A.; de Castro, E.R.; Senise, J.T.; Mourão, M.B.; Takano, C. Equipment for microwave enhanced carbothermic reductions. In Proceedings of the 2009 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC), Belem, Brazil, 3–6 November 2009; pp. 167–171. [Google Scholar]
- Gunnewiek, R.F.; Souto, P.M.; Kiminami, R.H. Synthesis of nanocrystalline boron carbide by direct microwave carbothermal reduction of boric acid. J. Nanomater. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.L. Microwave and plasma sintering of ceramics. Ceram. Int. 1991, 17, 295–300. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Kheradmandfard, M.; Minouei, H.; Tsvetkov, N.; Vayghan, A.K.; Kashani-Bozorg, S.F.; Kim, G.; Hong, S.I.; Kim, D.-E. Ultrafast green microwave-assisted synthesis of high-entropy oxide nanoparticles for Li-ion battery applications. Mater. Chem. Phys. 2021, 262, 124265. [Google Scholar] [CrossRef]
- Storr, B.; Moore, L.; Chakrabarty, K.; Mohammed, Z.; Rangari, V.; Chen, C.-C.; Catledge, S.A. Properties of high entropy borides synthesized via microwave-induced plasma. APL Mater. 2022, 10, 061109. [Google Scholar] [CrossRef]
- Storr, B.; Kodali, D.; Chakrabarty, K.; Baker, P.A.; Rangari, V.; Catledge, S.A. Single-step synthesis process for high-entropy transition metal boride powders using microwave plasma. Ceramics 2021, 4, 257–264. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.-H.; Ferreira, L.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Walle, A.; Tiwary, P.; De Jong, M.; Olmsted, D.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.-Q.; Liu, Z.-K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. J. Appl. Math. Mech./Z. Für Angew. Math. Und Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik:(mit Ausschluss der Kristalloptik); BG Teubner: Leipzig, Germany; Berlin, Germany, 1910; Volume 34. [Google Scholar]
- Tian, Y.; Xu, B.; Zhao, Z. Microscopic Theory of Hardness and Design of Novel Superhard Crystals. Int. J. Refract. Met. Hard Mater. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Wang, F.; Monteverde, F.; Cui, B. Will high-entropy carbides and borides be enabling materials for extreme environments? Int. J. Extrem. Manuf. 2023, 5, 022002. [Google Scholar] [CrossRef]
- Irfan, H.; Racik, K.M.; Anand, S. Microstructural evaluation of CoAl2O4 nanoparticles by Williamson–Hall and size–strain plot methods. J. Asian Ceram. Soc. 2018, 6, 54–62. [Google Scholar] [CrossRef] [Green Version]
- El Khouri, A.; Zegzouti, A.; Elaatmani, M.; Capitelli, F. Bismuth-substituted hydroxyapatite ceramics synthesis: Morphological, structural, vibrational and dielectric properties. Inorg. Chem. Commun. 2019, 110, 107568. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Processing of dense high-entropy boride ceramics. J. Eur. Ceram. Soc. 2020, 40, 3815–3823. [Google Scholar] [CrossRef]
- Ratzker, B.; Wagner, A.; Sokol, M.; Kalabukhov, S.; Frage, N. Stress-enhanced dynamic grain growth during high-pressure spark plasma sintering of alumina. Acta Mater. 2019, 164, 390–399. [Google Scholar] [CrossRef]
- Averback, R.; Höfler, H.; Hahn, H.; Logas, J. Sintering and grain growth in nanocrystalline ceramics. Nanostructured Mater. 1992, 1, 173–178. [Google Scholar] [CrossRef]
- Langer, J.; Hoffmann, M.J.; Guillon, O. Direct comparison between hot pressing and electric field-assisted sintering of submicron alumina. Acta Mater. 2009, 57, 5454–5465. [Google Scholar] [CrossRef]
- Shen, X.-Q.; Liu, J.-X.; Li, F.; Zhang, G.-J. Preparation and characterization of diboride-based high entropy (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)B2–SiC particulate composites. Ceram. Int. 2019, 45, 24508–24514. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef] [Green Version]
- Tallarita, G.; Licheri, R.; Garroni, S.; Barbarossa, S.; Orrù, R.; Cao, G. High-entropy transition metal diborides by reactive and non-reactive spark plasma sintering: A comparative investigation. J. Eur. Ceram. Soc. 2020, 40, 942–952. [Google Scholar] [CrossRef]
- Sitler, S.J.; Raja, K.S.; Charit, I. Hot corrosion behavior of ZrB2-HfB2 solid solutions in KCl and K2SO4 at 1500 °C. Ceram. Int. 2017, 43, 17071–17085. [Google Scholar] [CrossRef]
- Ghoshal, A.; Walock, M.J.; Nieto, A.; Murugan, M.; Hofmeister-Mock, C.; Pepi, M.; Bravo, L.; Wright, A.; Luo, J. Experimental Analysis and Material Characterization of Ultra High Temperature Composites. In Turbo Expo: Power for Land, Sea, and Air, Proceedings of the ASME Turbo Expo 2021: Turbomachinery Technical Conference and Exposition, Virtual, 7–11 June 2021; American Society of Mechanical Engineers: New York, NY, USA, 2021. [Google Scholar]
- Prasad, A.; Fotou, G.; Li, S. The effect of polymer hardness, pore size, and porosity on the performance of thermoplastic polyurethane-based chemical mechanical polishing pads. J. Mater. Res. 2013, 28, 2380–2393. [Google Scholar] [CrossRef] [Green Version]







| Synthesis Method | Excess Reducing Agents | Measured Lattice Constants | Unit Cell Volume | Theoretical Density * | |
|---|---|---|---|---|---|
| (wt%) | a (Ǻ) | c (Ǻ) | (Ǻ)3 | (g/cm3) (Relative Density) | |
| BCTR | 9% (B4C) | 3.09023 (6) | 3.35918 (6) | 27.78 | 8.40 (93.7%) |
| BTR | 10% (boron) | 3.07890 (6) | 3.34133 (6) | 27.43 | 8.51 (92.5%) |
| BCTR + FAST | - | 3.09901 (4) | 3.33501 (4) | 27.73 | 8.41(99.4%) |
| Synthesis Method | Crystallite Size (nm) | Microstrain ɛ (×10−3) |
|---|---|---|
| BCTR | 41.6 ± 17.9 | 1.26 ± 0.65 |
| BTR | 28.5 ± 10.5 | 1.69 ± 0.83 |
| BCTR + FAST | 64.9 ± 25.5 | 1.12 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storr, B.; Amezaga, C.; Moore, L.; Iwan, S.; Vohra, Y.K.; Chen, C.-C.; Catledge, S.A. High Entropy Borides Synthesized by the Thermal Reduction of Metal Oxides in a Microwave Plasma. Materials 2023, 16, 4475. https://doi.org/10.3390/ma16124475
Storr B, Amezaga C, Moore L, Iwan S, Vohra YK, Chen C-C, Catledge SA. High Entropy Borides Synthesized by the Thermal Reduction of Metal Oxides in a Microwave Plasma. Materials. 2023; 16(12):4475. https://doi.org/10.3390/ma16124475
Chicago/Turabian StyleStorr, Bria, Carolina Amezaga, Luke Moore, Seth Iwan, Yogesh K. Vohra, Cheng-Chien Chen, and Shane A. Catledge. 2023. "High Entropy Borides Synthesized by the Thermal Reduction of Metal Oxides in a Microwave Plasma" Materials 16, no. 12: 4475. https://doi.org/10.3390/ma16124475