Improving Anti-Coking Properties of Ni/Al2O3 Catalysts via Synergistic Effect of Metallic Nickel and Nickel Phosphides in Dry Methane Reforming
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalyst Performance Tests
3. Results and Discussion
3.1. Characterization of Fresh Catalysts
- -
- -
- -
3.2. Catalytic Performance
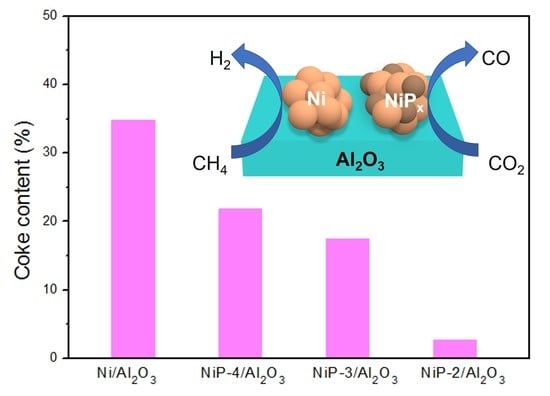
3.3. Characterization of Spent Catalysts
3.4. DRIFTS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashcroft, A.; Cheetham, A.; Green, M. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Arman, A.; Hagos, F.Y.; Abdullah, A.A.; Mamat, R.; Aziz, A.R.A.; Cheng, C.K. Syngas production through steam and CO2 reforming of methane over Ni-based catalyst-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 042032. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Hengyong, X.; Xixian, S.; Yemei, F.; Guolin, X.; Jinxiang, L.; Wenge, Y.; Peiheng, Z. Studies of Reforming Methane with Carbon Dioxide to Produce Synthesis Gas I. Catalyst and Its Catalytic Property. Petrochem. Technol. 1992, 3, 1. [Google Scholar]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile Route for Synthesizing Ordered Mesoporous Ni–Ce–Al Oxide Materials and Their Catalytic Performance for Methane Dry Reforming to Hydrogen and Syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Goula, M.; Lemonidou, A.; Efstathiou, A.M. Characterization of Carbonaceous Species Formed during Reforming of CH4 with CO2 over Ni/CaO–Al2O3 Catalysts Studied by Various Transient Techniques. J. Catal. 1996, 161, 626–640. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Shen, J.; Reule, A.A.C.; Semagina, N. Ni/MgAl2O4 catalyst for low-temperature oxidative dry methane reforming with CO2. Int. J. Hydrog. Energy 2019, 44, 4616–4629. [Google Scholar] [CrossRef]
- Zanganeh, R.; Rezaei, M.; Zamaniyan, A. Dry reforming of methane to synthesis gas on NiO–MgO nanocrystalline solid solution catalysts. Int. J. Hydrog. Energy 2013, 38, 3012–3018. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Mi, Y.; Yang, S.; Wang, Z.; Liu, W.; Wu, D.; Peng, H. Trifunctional strategy for the design and synthesis of a Ni-CeO2@SiO2 catalyst with remarkable low-temperature sintering and coking resistance for methane dry reforming. Chin. J. Catal. 2021, 42, 1808–1820. [Google Scholar] [CrossRef]
- Li, K.; Chang, X.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Assabumrungrat, S.; Zhao, Z.-J.; Zeng, L.; Gong, J. Ordered mesoporous Ni/La2O3 catalysts with interfacial synergism towards CO2 activation in dry reforming of methane. Appl. Catal. B Environ. 2019, 259, 118092. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Kong, W.; Jin, F.; Bai, J.; Zhang, J.; Sun, Y. Design of a carbon-resistant Ni@S-2 reforming catalyst: Controllable Ni nanoparticles sandwiched in a peasecod-like structure. Appl. Catal. B Environ. 2021, 282, 119546. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Costa, P.D. Syngas production from dry methane reforming over yttrium-promoted nickel-KIT-6 catalysts. Int. J. Hydrog. Energy 2019, 44, 274–286. [Google Scholar] [CrossRef]
- Han, B.; Wang, F.; Zhang, L.; Wang, Y.; Fan, W.; Xu, L.; Yu, H.; Li, Z. Syngas production from methane steam reforming and dry reforming reactions over sintering-resistant Ni@ SiO2 catalyst. Res. Chem. Intermed. 2020, 46, 1735–1748. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; Rivas, B.D.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158, 190–201. [Google Scholar] [CrossRef]
- Cho, E.; Lee, Y.-H.; Kim, H.; Jang, E.J.; Kwak, J.H.; Lee, K.; Ko, C.H.; Yoon, W.L. Ni catalysts for dry methane reforming prepared by A-site exsolution on mesoporous defect spinel magnesium aluminate. Appl. Catal. A Gen. 2020, 602, 117694. [Google Scholar] [CrossRef]
- Sierra Gallego, G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Dual Active-Site Mechanism for Dry Methane Reforming over Ni/La2O3 Produced from LaNiO3 Perovskite. Ind. Eng. Chem. Res. 2008, 47, 9272–9278. [Google Scholar] [CrossRef]
- Gallego, G.S.; Marín, J.G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Influence of Pr and Ce in dry methane reforming catalysts produced from La1−xAxNiO3−δ perovskites. Appl. Catal. A Gen. 2009, 369, 97–103. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, eabb1573. [Google Scholar] [CrossRef] [PubMed]
- Dębek, R.; Galvez, M.E.; Launay, F.; Motak, M.; Grzybek, T.; Costa, P.D. Low temperature dry methane reforming over Ce, Zr and CeZr promoted Ni–Mg–Al hydrotalcite-derived catalysts. Int. J. Hydrog. Energy 2016, 41, 11616–11623. [Google Scholar] [CrossRef] [Green Version]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Costa, P.D. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Liu, H.; Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Costa, P.D.; Gálvez, M.E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16. [Google Scholar] [CrossRef]
- Danghyan, V.; Kumar, A.; Mukasyan, A.; Wolf, E.E. An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane. Appl. Catal. B Environ. 2020, 273, 119056. [Google Scholar] [CrossRef]
- Padi, S.P.; Shelly, L.; Komarala, E.P.; Schweke, D.; Hayun, S.; Rosen, B.A. Coke-free methane dry reforming over nano-sized NiO-CeO2 solid solution after exsolution. Catal. Commun. 2020, 138, 105951. [Google Scholar] [CrossRef]
- Turap, Y.; Wang, I.; Fu, T.; Wu, Y.; Wang, Y.; Wang, W. Co–Ni alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 6538–6548. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.J.; Román-Martínez, M.C. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Sulfur-passivated nickel catalysts for carbon-free steam reforming of methane. J. Catal. 1984, 85, 31–43. [Google Scholar] [CrossRef]
- Brungs, A.J.; York, A.P.; Claridge, J.B.; Márquez-Alvarez, C.; Green, M.L. Dry reforming of methane to synthesis gas over supported molybdenum carbide catalysts. Catal. Lett. 2000, 70, 117–122. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Zhu, Y.; Qiu, J.; Au, C. Catalytic role of β-Mo2C in DRM catalysts that contain Ni and Mo. Catal. Today 2015, 258, 676–683. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, S.; Li, X.; Zhang, A.; Shi, M.; Zhu, Y.; Qiu, J.; Au, C. Synergism in NiMoOx precursors essential for CH4/CO2 dry reforming. Catal. Today 2014, 233, 46–52. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, W. Effect of cobalt (nickel) content on the catalytic performance of molybdenum carbides in dry-methane reforming. Fuel Processing Technol. 2010, 91, 185–193. [Google Scholar] [CrossRef]
- Yao, Z.; Luan, F.; Sun, Y.; Jiang, B.; Song, J.; Wang, H. Molybdenum phosphide as a novel and stable catalyst for dry reforming of methane. Catal. Sci. Technol. 2016, 6, 7996–8004. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Q.; Yao, Z.; Dou, B.; Shi, Y.; Sun, Y. A comparative study of molybdenum phosphide catalyst for partial oxidation and dry reforming of methane. Int. J. Hydrog. Energy 2019, 44, 11441–11447. [Google Scholar] [CrossRef]
- González-Castaño, M.; Saché, E.L.; Berry, C.; Pastor-Pérez, L.; Arellano-García, H.; Wang, Q.; Reina, T.R. Nickel Phosphide Catalysts as Efficient Systems for CO2 Upgrading via Dry Reforming of Methane. Catalysts 2021, 11, 446. [Google Scholar] [CrossRef]
- Bang, S.; Hong, E.; Baek, S.W.; Shin, C.-H. Effect of acidity on Ni catalysts supported on P-modified Al2O3 for dry reforming of methane. Catal. Today 2018, 303, 100–105. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Chu, W.; Wei, F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming. Catal. Sci. Technol. 2016, 6, 3594–3605. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Nickel Phosphide/Silica Catalysts for the Gas-Phase Hydrogenation of Furfural to High–Added–Value Chemicals. ChemCatChem 2017, 9, 2881–2889. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Q.; Shi, Y.; Yao, Z.; Ding, W.; Dou, B. Binary and ternary transition metal phosphides for dry reforming of methane. React. Chem. Eng. 2020, 5, 719–727. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Highly efficient NiAl2O4-free Ni/γ-Al2O3 catalysts prepared by solution combustion method for CO methanation. Fuel 2014, 137, 155–163. [Google Scholar] [CrossRef]
- Li, T.; Virginie, M.; Khodakov, A.Y. Effect of potassium promotion on the structure and performance of alumina supported carburized molybdenum catalysts for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2017, 542, 154–162. [Google Scholar] [CrossRef]
- Youn, M.H.; Seo, J.G.; Kim, P.; Song, I.K. Role and effect of molybdenum on the performance of Ni-Mo/γ-Al2O3 catalysts in the hydrogen production by auto-thermal reforming of ethanol. J. Mol. Catal. A Chem. 2007, 261, 276–281. [Google Scholar] [CrossRef]
- Malaibari, Z.O.; Croiset, E.; Amin, A.; Epling, W. Effect of interactions between Ni and Mo on catalytic properties of a bimetallic Ni-Mo/Al2O3 propane reforming catalyst. Appl. Catal. A Gen. 2015, 490, 80–92. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Yoo, J.; Park, S.; Choi, J.H.; Lee, Y.J.; Song, J.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous nickel–phosphorus–alumina aerogel catalyst. Int. J. Hydrog. Energy 2014, 39, 4909–4916. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, N.; Chu, W.; Deng, J.; Luo, S. In situ controllable assembly of layered-double-hydroxide-based nickel nanocatalysts for carbon dioxide reforming of methane. Catal. Sci. Technol. 2015, 5, 1588–1597. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal–support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Galetti, A.E.; Gomez, M.F.; Arrúa, L.A.; Abello, M.C. Hydrogen production by ethanol reforming over NiZnAl catalysts: Influence of Ce addition on carbon deposition. Appl. Catal. A Gen. 2008, 348, 94–102. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B Environ. 2020, 265, 118538. [Google Scholar] [CrossRef]
- Coenen, K.; Gallucci, F.; Mezari, B.; Hensen, E.; Annaland, M.V.S. An in-situ IR study on the adsorption of CO2 and H2O on hydrotalcites. J. CO2 Util. 2018, 24, 228–239. [Google Scholar] [CrossRef]
- Quindimil, A.; Onrubia-Calvo, J.A.; Davó-Quiñonero, A.; Bermejo-López, A.; Bailón-García, E.; Pereda-Ayo, B.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Intrinsic kinetics of CO2 methanation on low-loaded Ni/Al2O3 catalyst: Mechanism, model discrimination and parameter estimation. J. CO2 Util. 2022, 57, 101888. [Google Scholar] [CrossRef]
- Proaño, L.; Tello, E.; Arellano-Trevino, M.A.; Wang, S.; Farrauto, R.J.; Cobo, M. In-situ DRIFTS study of two-step CO2 capture and catalytic methanation over Ru,“Na2O”/Al2O3 Dual Functional Material. Appl. Surf. Sci. 2019, 479, 25–30. [Google Scholar] [CrossRef]
- Zhou, M.; Andrews, L. Infrared spectra of the CO2− and C2O4− anions isolated in solid argon. J. Chem. Phys. 1999, 110, 2414–2422. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Danon, A.; K.Vijayan, B.; Gray, K.A.; Stair, P.C.; Weitz, E. Role of the Surface Lewis Acid and Base Sites in the Adsorption of CO2 on Titania Nanotubes and Platinized Titania Nanotubes: An in Situ FT-IR Study. J. Phys. Chem. C 2013, 117, 12661–12678. [Google Scholar] [CrossRef]
- Morales, A.; Agudelo, M.M.R.D.; Hernández, F. Adsorption mechanism of phosphorus on alumina. Appl. Catal. 1988, 41, 261–271. [Google Scholar] [CrossRef]
- Lewis, J.M.; Kydd, R.A. Adsorption mechanism of phosphoric acid on γ-alumina. J. Catal. 1991, 132, 465–471. [Google Scholar] [CrossRef]
- Veen, J.A.R.H.V.; Hendriks, P.A.J.M.; Andrea, R.R.; Romers, E.J.G.M.; Wilson, A.E. Chemistry of phosphomolybdate adsorption on alumina surfaces. 2. The molybdate/phosphated alumina and phosphomolybdate/alumina systems. J. Phys. Chem. 1990, 94, 5282–5285. [Google Scholar] [CrossRef]










| Catalyst | Chemical Composition | Textural Properties | Size b (nm) | ||||
|---|---|---|---|---|---|---|---|
| Ni a (wt.%) | P a (wt.%) | Ni/P Molar Ratio | SBET (m2/g) | Vpore (cm3/g) | Dpore (nm) | ||
| Ni/Al2O3 | 9.56 | - | - | 156 | 0.66 | 13.33 | 9.4 ± 2.1 |
| NiP-4/Al2O3 | 9.81 | 1.30 | 3.97 | 171 | 0.68 | 13.50 | 9.0 ± 1.6 |
| NiP-3/Al2O3 | 8.56 | 1.43 | 3.17 | 167 | 0.66 | 12.61 | 8.2 ± 1.5 |
| NiP-2/Al2O3 | 10.0 | 2.54 | 2.04 | 162 | 0.64 | 13.19 | 7.7 ± 1.6 |
| Catalyst | Nickel Species Distribution | |||
|---|---|---|---|---|
| Ni0 | Niδ+ | Ni2+ | ||
| Ni/Al2O3 | Position (eV) | 852.1 | - | 855.3 |
| Species distribution | 29.1 | 0 | 70.9 | |
| NiP-4/Al2O3 | Position (eV) | 852.2 | 853.1 | 856.5 |
| Species distribution | 18.8 | 7.7 | 73.5 | |
| NiP-3/Al2O3 | Position (eV) | 852.3 | 853.2 | 856.3 |
| Species distribution | 26.8 | 18.5 | 54.7 | |
| NiP-2/Al2O3 | Position (eV) | 852.2 | 853.2 | 856.2 |
| Species distribution | 32.0 | 32.4 | 35.6 | |
| Catalyst | Particle Size Distribution (nm) | Particle Size Enhancing Range (%) | |
|---|---|---|---|
| Fresh Catalysts | Spent Catalysts | ||
| Ni/Al2O3 | 9.4 ± 2.1 | 10.3 ± 2.5 | 9.6 |
| NiP-4/Al2O3 | 9.0 ± 1.6 | 9.5 ± 1.5 | 5.6 |
| NiP-3/Al2O3 | 8.2 ± 1.5 | 8.5 ± 1.4 | 3.7 |
| NiP-2/Al2O3 | 7.7 ± 1.6 | 8.4 ± 1.8 | 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Wang, S.; Li, Y.; Yang, F.; Yu, H.; Chu, Y.; Li, T.; Yin, H. Improving Anti-Coking Properties of Ni/Al2O3 Catalysts via Synergistic Effect of Metallic Nickel and Nickel Phosphides in Dry Methane Reforming. Materials 2022, 15, 3044. https://doi.org/10.3390/ma15093044
Shi Y, Wang S, Li Y, Yang F, Yu H, Chu Y, Li T, Yin H. Improving Anti-Coking Properties of Ni/Al2O3 Catalysts via Synergistic Effect of Metallic Nickel and Nickel Phosphides in Dry Methane Reforming. Materials. 2022; 15(9):3044. https://doi.org/10.3390/ma15093044
Chicago/Turabian StyleShi, Yu, Shiwei Wang, Yiming Li, Fan Yang, Hongbo Yu, Yuting Chu, Tong Li, and Hongfeng Yin. 2022. "Improving Anti-Coking Properties of Ni/Al2O3 Catalysts via Synergistic Effect of Metallic Nickel and Nickel Phosphides in Dry Methane Reforming" Materials 15, no. 9: 3044. https://doi.org/10.3390/ma15093044