Assembly of Semiconductor Nanorods into Circular Arrangements Mediated by Block Copolymer Micelles
Abstract
:1. Introduction
2. Materials and Methods
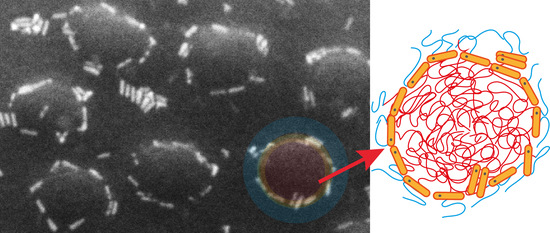
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bockstaller, M.R.; Thomas, E.L. Optical properties of polymer-based photonic nanocomposite materials. J. Phys. Chem. B 2003, 107, 10017–10024. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Composto, R.J. Nanorod self-assembly for tuning optical absorption. ACS Nano 2010, 4, 6941–6949. [Google Scholar] [CrossRef] [PubMed]
- Stebe, K.J.; Lewandowski, E.; Ghosh, M. Oriented assembly of metamaterials. Science 2009, 325, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P. Self-assembly of inorganic nanocrystals: Fabrication and collective intrinsic properties. Acc. Chem. Res. 2007, 40, 685–693. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Composto, R.J. Functional polymer nanocomposites enhanced by nanorods. Macromolecules 2014, 47, 875–887. [Google Scholar] [CrossRef]
- Wang, D.; Hore, M.J.A.; Ye, X.; Zheng, C.; Murray, C.B.; Composto, R.J. Gold nanorod length controls dispersion, local ordering, and optical absorption in polymer nanocomposite films. Soft Matter 2014, 10, 3404–3413. [Google Scholar] [CrossRef]
- Jiang, G.; Hore, M.J.A.; Gam, S.; Composto, R.J. Gold nanorods dispersed in homopolymer films: Optical properties controlled by self-assembly and percolation of nanorods. ACS Nano 2012, 6, 1578–1588. [Google Scholar] [CrossRef]
- Deshmukh, R.D.; Liu, Y.; Composto, R.J. Two-dimensional confinement of nanorods in block copolymer domains. Nano Lett. 2007, 7, 3662–3668. [Google Scholar] [CrossRef]
- Ploshnik, E.; Salant, A.; Banin, U.; Shenhar, R. Hierarchical surface patterns of nanorods obtained by co-assembly with block copolymers in ultrathin Films. Adv. Mater. 2010, 22, 2774–2779. [Google Scholar] [CrossRef]
- Ploshnik, E.; Salant, A.; Banin, U.; Shenhar, R. Co-assembly of block copolymers and nanorods in ultrathin films: Effects of copolymer size and nanorod filling fraction. Phys. Chem. Chem. Phys. 2010, 12, 11885–11893. [Google Scholar] [CrossRef]
- Halevi, A.; Halivni, S.; Oded, M.; Müller, A.H.E.; Banin, U.; Shenhar, R. Co-assembly of A-B diblock copolymers with B’-type nanoparticles in thin films: Effect of copolymer composition and nanoparticle shape. Macromolecules 2014, 47, 3022–3032. [Google Scholar] [CrossRef]
- Thomas, K.G.; Barazzouk, S.; Ipe, B.I.; Joseph, S.T.S.; Kamat, P.V. Uniaxial plasmon coupling through longitudinal self-assembly of gold nanorods. J. Phys. Chem. B 2004, 108, 13066–13068. [Google Scholar] [CrossRef]
- Nie, Z.H.; Fava, D.; Rubinstein, M.; Kumacheva, E. “Supramolecular” assembly of gold nanorods end-terminated with polymer “Pom-Poms”: Effect of pom-pom structure on the association modes. J. Am. Chem. Soc. 2008, 130, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, S.S.; Il-Yoo, S.; Sohn, B.H. Dichroic plasmon superstructures of Au nanorods over macroscopic areas via directed self-assemblies of diblock copolymers. Adv. Mater. Interfaces 2019, 6, 1901257. [Google Scholar] [CrossRef]
- Wang, T.; Zhuang, J.Q.; Lynch, J.; Chen, O.; Wang, Z.L.; Wang, X.R.; LaMontagne, D.; Wu, H.M.; Wang, Z.W.; Cao, Y.C. Self-assembled colloidal superparticles from nanorods. Science 2012, 338, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, R.A.; Menumerov, E.; Neretina, S. When lithography meets self-assembly: A review of recent advances in the directed assembly of complex metal nanostructures on planar and textured surfaces. Nanotechnology 2017, 28, 282002. [Google Scholar] [CrossRef]
- Von Freymann, G.; Ledermann, A.; Thiel, M.; Staude, I.; Essig, S.; Busch, K.; Wegener, M. Three-dimensional nanostructures for photonics. Adv. Funct. Mater. 2010, 20, 1038–1052. [Google Scholar] [CrossRef]
- Roller, E.M.; Khorashad, L.K.; Fedoruk, M.; Schreiber, R.; Govorov, A.O.; Liedl, T. DNA-assembled nanoparticle rings exhibit electric and magnetic resonances at visible frequencies. Nano Lett. 2015, 15, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.B.; Lu, X.Y.; Wang, W.Y.; Kang, N.G.; Mays, J.W. Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Kim, H.-C.; Park, S.-M.; Hinsberg, W.D. Block copolymer based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef]
- Albert, J.N.L.; Epps, T.H., III. Self-assembly of block copolymer thin films. Mater. Today 2010, 13, 24–33. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Gupta, S.; Emrick, T.; Russell, T.P. Surface-functionalized CdSe nanorods for assembly in diblock copolymer templates. J. Am. Chem. Soc. 2006, 128, 3898–3899. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Borca-Tasciuc, T.; Plawsky, J. Controlling directed self-assembly of gold nanorods in patterned PS-b-PMMA thin films. Nanotechnology 2015, 26, 055301. [Google Scholar] [CrossRef]
- Rodríguez-Hernandez, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Cameron, N.S.; Corbierre, M.K.; Eisenberg, A. 1998 E.W.R. Steacie award lecture asymmetric amphiphilic block copolymers in solution: A morphological wonderland. Can. J. Chem. 1999, 77, 1311–1326. [Google Scholar] [CrossRef]
- Tuzar, Z.; Stepanek, P.; Konak, C.; Kratochvil, P. Block copolymer micelles near critical conditions. J. Colloid Interface Sci. 1985, 105, 372–377. [Google Scholar] [CrossRef]
- Gohy, J.F. Block copolymer micelles. Adv. Polym. Sci. 2005, 190, 65–136. [Google Scholar] [CrossRef]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Spatz, J.P.; Mössmer, S.; Hartmann, C.; Möller, M.; Herzog, T.; Krieger, M.; Boyen, H.G.; Ziemann, P.; Kabius, B. Ordered deposition of inorganic clusters from micellar block copolymer films. Langmuir 2000, 16, 407–415. [Google Scholar] [CrossRef]
- Spatz, J.P.; Roescher, A.; Moller, M. Gold nanoparticles in micellar poly(styrene)-b-poly(ethylene oxide) films-size and interparticle distance control in monoparticulate films. Adv. Mater. 1996, 8, 337–340. [Google Scholar] [CrossRef]
- Spatz, J.P.; Sheiko, S.; Moller, M. Ion-stabilized block copolymer micelles: Film formation and intermicellar interaction. Macromolecules 1996, 29, 3220–3226. [Google Scholar] [CrossRef]
- Spatz, J.P.; Mössmer, S.; Möller, M. Mineralization of gold nanoparticles in a block copolymer microemulsion. Chem. Eur. J. 1996, 2, 1552–1555. [Google Scholar] [CrossRef]
- Seregina, M.V.; Bronstein, L.M.; Platonova, O.A.; Chernyshov, D.M.; Valetsky, P.M.; Hartmann, J.; Wenz, E.; Antonietti, M. Preparation of noble-metal colloids in block copolymer micelles and their catalytic properties in hydrogenation. Chem. Mater. 1997, 9, 923–931. [Google Scholar] [CrossRef]
- Möller, M.; Spatz, J.P. Mineralization of nanoparticles in block copolymer micelles. Curr. Opin. Colloid Interface Sci. 1997, 2, 177–187. [Google Scholar] [CrossRef]
- Gao, Z.S.; Eisenberg, A. A model of micellization for block-copolymers in solutions. Macromolecules 1993, 26, 7353–7360. [Google Scholar] [CrossRef]
- Liang, W.J.; Lien, C.H.; Kuo, P.L. Preparation of platinum nanoparticles in salt-induced micelles of dumbbell-shaped ABA copolymer. J. Colloid Interface Sci. 2006, 294, 371–375. [Google Scholar] [CrossRef]
- Mayer, A.B.R.; Mark, J.E.; Morris, R.E. Palladium and platinum nanocatalysts protected by amphiphilic block copolymers. Polym. J. 1998, 30, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Antonietti, M.; Wenz, E.; Bronstein, L.; Seregina, M. Synthesis and characterization of noble metal colloids in block copolymer micelles. Adv. Mater. 1995, 7, 1000–1005. [Google Scholar] [CrossRef]
- Loginova, T.P.; Lykhina, O.V.; Yudanova, E.A.; Khotina, I.A.; Timofeeva, G.I.; Lependina, O.L.; Volkov, V.V.; Dembo, K.A.; Solodovnikov, S.P. Synthesis and characterization of cobalt ferrite nanoparticles in hybrid micelles of poly styrene-block-(ethylene oxide) and sodium dodecyl sulfate. Polym. Sci. Ser. A 2010, 52, 849–855. [Google Scholar] [CrossRef]
- Samal, M.; Mohapatra, P.R.; Yun, K.S. Controlled growth of CdS quantum dot in an amphiphilic diblock copolymer poly(2-vinyl pyridine)-b-poly(n-hexyl isocyanate) reversed micelle nanoreactor. J. Nanosci. Nanotechnol. 2015, 15, 6779–6783. [Google Scholar] [CrossRef]
- Sohn, B.H.; Choi, J.M.; Yoo, S.I.; Yun, S.H.; Zin, W.C.; Jung, J.C.; Kanehara, M.; Hirata, T.; Teranishi, T. Directed self-assembly of two kinds of nanoparticles utilizing monolayer films of diblock copolymer micelles. J. Am. Chem. Soc. 2003, 125, 6368–6369. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Hong, R.; Emrick, T.; Walker, G.C. Ordered CdSe Nanoparticles within self-assembled block copolymer domains on surfaces. Langmuir 2007, 23, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Kumar, S.; Lee, A.; Felorzabihi, N.; Shen, L.; Zhao, F.; Froimowicz, P.; Scholes, G.D.; Winnik, M.A. Nanoscale co-organization of quantum dots and conjugated polymers using polymeric micelles as templates. J. Am. Chem. Soc. 2008, 130, 9481–9491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, M.F.; He, S.; Qian, J.S.; Saffari, A.; Lee, A.; Kumar, S.; Hassan, Y.; Guenther, A.; Scholes, G.; et al. Sphere-to-wormlike network transition of block copolymer micelles containing CdSe quantum dots in the corona. Macromolecules 2010, 43, 5066–5074. [Google Scholar] [CrossRef]
- Ku, K.H.; Kim, M.P.; Paek, K.; Shin, J.M.; Chung, S.; Jang, S.G.; Chae, W.S.; Yi, G.R.; Kim, B.J. Multicolor emission of hybrid block copolymer-quantum dot microspheres by controlled spatial isolation of quantum dots. Small 2013, 9, 2667–2672. [Google Scholar] [CrossRef]
- Nie, X.B.; Cui, J.; Jiang, W. Ultralong cylindrical micelles precisely located with semiconductor nanorods by solvent evaporation-driven self-assembly. Soft Matter 2014, 10, 8051–8059. [Google Scholar] [CrossRef]
- Nie, X.B.; Jiang, W. Luminous block copolymer-quantum dots hybrids formed by cooperative assembly in a selective solvent. RSC Adv. 2014, 4, 19613–19621. [Google Scholar] [CrossRef]
- Rafipoor, M.; Schmidtke, C.; Wolter, C.; Strelow, C.; Weller, H.; Lange, H. Clustering of CdSe/CdS quantum dot/quantum rods into micelles can form bright, non-blinking, stable, and biocompatible probes. Langmuir 2015, 31, 9441–9447. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.S.; Ji, X.L. Influence of mixed common solvent on the co-assembled morphology of PS-b-PEO and CdS quantum dots. Chin. J. Polym. Sci. 2016, 34, 1079–1090. [Google Scholar] [CrossRef]
- Kim, H.; Lim, Y.; Kim, S.; Kim, S.S.; Sohn, B.H. nanoscale arrangement of diblock copolymer micelles with Au nanorods. Nanotechnology 2014, 25, 455602. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Adel, P.; Bloh, J.; Hinrichs, D.; Kodanek, T.; Dorfs, D. Determination of all dimensions of CdSe seeded CdS nanorods solely via their UV/vis spectra. Z. Phys. Chem. 2017, 231, 93–106. [Google Scholar] [CrossRef]
- Singh, S.; Samanta, P.; Srivastava, R.; Horechyy, A.; Reuter, U.; Stamm, M.; Chen, H.L.; Nandan, B. Ligand displacement induced morphologies in block copolymer/quantum dot hybrids and formation of core-shell hybrid nanoobjects. Phys. Chem. Chem. Phys. 2017, 19, 27651–27663. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Wang, D.; Fan, X.N.; Buriak, J.M. Assembly of aligned linear metallic patterns on silicon. Nat. Nanotechnol. 2007, 2, 500–506. [Google Scholar] [CrossRef]
- Lin, Y.; Böker, A.; He, J.B.; Sill, K.; Xiang, H.Q.; Abetz, C.; Li, X.F.; Wang, J.; Emrick, T.; Long, S.; et al. Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature 2005, 434, 55–59. [Google Scholar] [CrossRef]
- Ploshnik, E.; Langner, K.M.; Halevi, A.; Ben-Lulu, M.; Müller, A.H.E.; Fraaije, J.G.E.M.; Agur Sevink, G.J.; Shenhar, R. Hierarchical structuring in block copolymer nanocomposites through two phase-separation processes operating on different time scales. Adv. Funct. Mater. 2013, 23, 4215–4226. [Google Scholar] [CrossRef]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Kennemur, J.G. Poly(vinylpyridine) segments in block copolymers: Synthesis, self-assembly, and versatility. Macromolecules 2019, 52, 1354–1370. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzaffar-Kawasma, R.; Oded, M.; Shenhar, R. Assembly of Semiconductor Nanorods into Circular Arrangements Mediated by Block Copolymer Micelles. Materials 2022, 15, 2949. https://doi.org/10.3390/ma15082949
Muzaffar-Kawasma R, Oded M, Shenhar R. Assembly of Semiconductor Nanorods into Circular Arrangements Mediated by Block Copolymer Micelles. Materials. 2022; 15(8):2949. https://doi.org/10.3390/ma15082949
Chicago/Turabian StyleMuzaffar-Kawasma, Riham, Meirav Oded, and Roy Shenhar. 2022. "Assembly of Semiconductor Nanorods into Circular Arrangements Mediated by Block Copolymer Micelles" Materials 15, no. 8: 2949. https://doi.org/10.3390/ma15082949