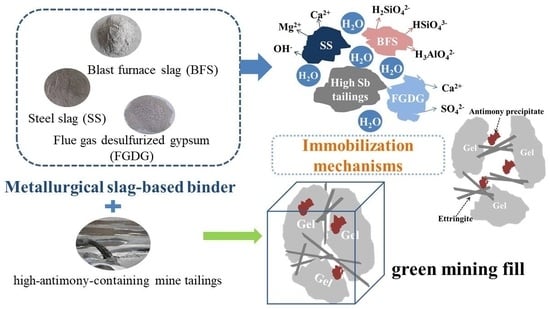
Study on Solidification and Stabilization of Antimony-Containing Tailings with Metallurgical Slag-Based Binders
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Materials
2.2. Mixture Proportion of Mining Fill Samples
2.3. Experimental Procedures
3. Results
3.1. UCS Test Results
3.2. Toxicity Characteristic Leaching Test (HJ 557-2010)
3.3. Microscopic Analysis
3.4. XRD Results
3.5. FT-IR Results
3.6. XPS Spectra
4. Discussions
5. Conclusions
- Green mining fill samples (MBT) exhibited higher strength and a more pronounced antimony fixation effect than those of OPC.
- No newly formed antimony-containing mineral phase was detected in the metallurgical slag, but it was mainly surrounded by the adsorbed gel.
- Even though the heavy metal antimony was found to be able to reduce the degree of polymerization in the gel, its influence on ettringite and its relationship are not yet proven.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishad, P.A.; Bhaskarapillai, N. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef]
- Ashley, P.M.; Craw, D.; Graham, B.P.; Chappell, D.A. Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. J. Geochem. Explor. 2003, 77, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, C.X.; Gong, D.X.; Deng, Y.C.; Huang, Y.; Zheng, J.F.; Xiong, S.; Tang, R.D.; Wang, Y.C.; Su, L. A review of the environmental chemical behavior, detection and treatment of antimony. Environ. Technol. Innov. 2021, 24, 102026. [Google Scholar] [CrossRef]
- Corneils, G.; Gerven, T.V.; Vandecasteele, C. Antimony leaching from MSWI bottom ash: Modelling of the effect of pH and carbonation. Waste Manag. 2012, 32, 278–286. [Google Scholar] [CrossRef]
- Guo, J.L.; Yin, Z.P.; Zhong, W.; Jing, C.Y. Immobilization and transformation of co-existing arsenic and antimony in highly contaminated sediment by nano zero-valent iron. J. Environ. Sci. 2022, 112, 152–160. [Google Scholar] [CrossRef]
- Xiao, B.L.; Wen, Z.J.; Miao, S.J. Utilization of steel slag for cemented tailings backfill: Hydration, strength, pore structure, and cost analysis. Case Stud. Constr. Mater. 2021, 15, e00621. [Google Scholar] [CrossRef]
- Almas, A.R.; Pironin, E.; Okkenhaug, G. The partitioning of Sb in contaminated soils after being immobilization by Fe-based amendments is more dynamic compared to Pb. Appl. Geochem. 2019, 108, 104378. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 Emissions from Cement Production. Earth Syst. Sci. Data Discuss. 2018, 10, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Han, R.; Yu, B.Y.; Wei, Y.M. Accounting process-related CO2 emissions from global cement production under Shared Socioeconomic Pathways. J. Clean. Prod. 2018, 184, 451–465. [Google Scholar] [CrossRef]
- Li, J.S.; Chen, L.; Zhan, B.J.; Wang, L.; Poon, C.S.; Tsang, D.C.W. Sustainable stabilization/solidification of arsenic-containing soil by blast slag and cement blends. Chemosphere 2021, 271, 129868. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Cho, D.W.; Tsang, D.C.W.; Yang, J.; Hou, D.Y.; Baek, K.; Kua, H.W.; Poon, C.S. Novel synergy of Si-rich minerals and reactive MgO for stabilisation/solidification of contaminated sediment. J. Hazard. Mater. 2019, 365, 695–706. [Google Scholar] [CrossRef]
- Salihoglu, G. Immobilization of antimony waste slag by applying geopolymerization and stabilization/solidification technologies. J. Air Waste Manag. Assoc. 2014, 64, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Etschmann, B.; Gerven, T.V.; Vandecasteenle, C. Mechanisms and modelling of antimonate leaching in hydrated cement paste suspensions. Cem. Concr. Res. 2012, 42, 1307–1316. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.F.; Zhang, S.Q.; Zhang, Y.Y.; Fu, P.F.; Yang, H.F.; Ni, W. Enhancing Arsenic Solidification/Stabilisation Efficiency of Metallurgical Slag-Based Green Mining Fill and Its Structure Analysis. Metals 2021, 11, 1389. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.F.; Zhang, S.Q.; Zhang, Y.Y.; Teng, G.X.; Li, X.Q.; Ni, W. Solidification/Stabilization of Arsenic-Containing Tailings by Steel Slag-Based Binders with High Efficiency and Low Carbon Footprint. Materials 2021, 14, 5864. [Google Scholar] [CrossRef] [PubMed]
- Rasaki, S.A.; Zhang, B.X.; Guarecuco, R.; Thomas, T.J.; Yang, M.H. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.Q.; Wang, Q.; Ni, W.; Li, K.Q.; Fu, P.F.; Hu, W.T.; Li, Z.F. Feasibility of using fly ash–slag-based binder for mine backfilling and its_associated leaching risks. J. Hazard. Mater. 2020, 400, 123191. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ni, W.; Gao, W.; Zhang, Y.Y.; Yan, Q.H.; Zhang, S.Q. Corrosion evaluation of steel slag based on a leaching solution test. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 790–801. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Gerven, T.V.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Gao, W.; Ni, W.; Zhang, Y.Y.; Li, Y.Y.; Shi, T.Y.; Li, Z.F. Investigation into the semi-dynamic leaching characteristics of arsenic and antimony from solidified/stabilized tailings using metallurgical slag-based binders. J. Hazard. Mater. 2020, 381, 120992. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, S.Q.; Ni, W.; Yan, Q.H.; Gao, W.; Li, Y.Y. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Gao, W.; Ni, W.; Zhang, S.Q.; Li, Y.Y.; Wang, K.; Huang, X.H.; Fu, P.F.; Hu, W.T. Influence of calcium hydroxide addition on arsenic leaching and solidification/stabilisation behaviour of metallurgical-slag-based green mining fill. J. Hazard. Mater. 2020, 390, 122161. [Google Scholar] [CrossRef]
- Yan, Q.H.; Ni, W.; Gao, W.; Li, Y.Y.; Zhang, Y.Y. Mechanism for solidification of arsenic with blast furnace slag-steel slag based cementitious materials. J. Cent. South Univ. (Sci. Technol.) 2019, 50, 1544–1550. [Google Scholar] [CrossRef]
- Li, Y.C.; Wu, J.X.; Hu, W.; Ren, B.Z.; Hursthouse, A.S. A mechanistic analysis of the influence of iron-oxidizing bacteria on antimony (V) removal from water by microscale zero-valent iron. J. Chem. Technol. Biotechnol. 2018, 93, 2527–2534. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.P.; Cheng, H.G.; Wang, J.S.; Wan, Y.F.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qiao, C.y.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, W.; Huang, X.Y.; Ma, X.M.; Li, D.Z. Characteristics of hydration and hardening of red mud of bayer process in carbide slag-flue gas desulfurization gypsum system. Mater. Rep. 2016, 30, 120–124. [Google Scholar] [CrossRef]
- Ni, W.; Li, Y.; Xu, D.; Xu, C.W.; Jiang, Y.Q.; Gao, G.J. Hydration mechanism of blast furnace slag-reduction slag based solid waste cementing materials. J. Cent. South Univ. (Sci. Technol.) 2019, 50, 2342–2351. [Google Scholar] [CrossRef]
- Eskander, S.B.; Bayoumi, T.A.; Tawfik, M.E. Immobilization of Borate Waste Simulate in Cement-Water Extended Polyester Composite Based on Poly(Ethylene Terephthalate) Waste. Polym.-Plast. Technol. Eng. 2006, 45, 939–945. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wu, P.Q.; Qiu, J.P.; Guo, Z.B.; Tian, Y.S.; Sun, X.G.; Gu, X.W. Recycling hazardous steel slag after thermal treatment to produce a binder for cemented paste backfill. Powder Technol. 2022, 395, 652–662. [Google Scholar] [CrossRef]
- Ines, G.L.; Fernandez-Jimenez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol-Gel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Noam, A. Covalent radii from ionization energies of isoelectronic series. Chem. Phys. Lett. 2014, 595–596, 214–219. [Google Scholar] [CrossRef]
- Santamaria, L.; Vicente, M.A.; Korili, S.A.; Gil, A. Effect of the preparation method and metal content on the synthesis of metal modified titanium oxide used for the removal of salicylic acid under UV light. Environ. Technol. 2018, 41, 2073–2084. [Google Scholar] [CrossRef]
- Li, Y.; Wu, B.H.; Ni, W.; Li, X.M. Synergies in Early Hydration Reaction of Slag-Steel Slag-Gypsum System. J. Northeast. Univ. (Nat. Sci.) 2020, 41, 581–586. [Google Scholar] [CrossRef]
- Avalos, N.M.; Varga, T.; Mergelsberg, S.T.; Silverstein, J.A.; Saslow, A.A. Behavior of iodate substituted ettringite during aqueous leaching. Appl. Geochem. 2021, 125, 104863. [Google Scholar] [CrossRef]
- Pointeau, I.; Reiller, P.; Mace, N.; Landesman, C.; Coreau, N. Measurement and modeling of the surface potential evolution of hydrated cement pastes as a function of degradation. J. Colloid Interface Sci. 2006, 300, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Long, G.C.; Zheng, X.H.; Ma, C.; Xie, Y.J.; Sun, Z.P. Water evolution and hydration kinetics of cement paste under steam-curing condition based on low-field NMR method. Constr. Build. Mater. 2020, 271, 121583. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Ochs, M.; Pointeau, I.; Giffaut, E. Caesium sorption by hydrated cement as a function of degradation state: Experiments and modelling. Waste Manag. 2006, 26, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Gerven, T.V.; Snellings, R.; Verbinnen, B.; Elsen, J.; Vandecasteele, C. Stability of pyrochlores in alkaline matrices Solubility of calcium antimonate. Appl. Geochem. 2011, 26, 809–817. [Google Scholar] [CrossRef]









| Materials | Sb-MT | BFS | SS | FGDG | |
|---|---|---|---|---|---|
| Chemical composition | MgO | 0.99 | 8.94 | 6.00 | 1.04 |
| Oxide (wt. %) | Al2O3 | 4.45 | 15.43 | 6.24 | 0.78 |
| SiO2 | 40.34 | 24.76 | 18.16 | 2.03 | |
| SO3 | 17.31 | 0.83 | 0.29 | 44.97 | |
| CaO | 15.15 | 46.16 | 42.58 | 45.31 | |
| Fe2O3 | 17.52 | 2.52 | 17.66 | 0.48 | |
| Blaine fineness (m2/Kg) | - | 400 | 400 | 360 | |
| pH | 7.35 | 11.92 | 12.28 | 7.85 | |
| Leaching Sb concentration (μg/L) | 524 | ND | ND | ND | |
| Notation | MSB (Mass Fraction/wt. %) | Binders/Tailings (w/w) | Solid Concentration a (wt. %) | ||
|---|---|---|---|---|---|
| BFS | SS | FGDG | |||
| MBT | 60 | 30 | 10 | ¼ | 86 |
| OPC b | 100(OPC) | ||||
| Elements | Ca | Al | Si | O | S |
|---|---|---|---|---|---|
| Blank group | 347.30 | 74.26 | 102.10 | 531.67 | 168.99 |
| Sb cured sample | 347.11 | 74.26 | 102.00 | 531.54 | 169.21 |
| Changes | −0.19 | 0 | −0.10 | −0.13 | +0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ni, W.; Gao, W.; Zhang, S.; Fu, P.; Li, Y. Study on Solidification and Stabilization of Antimony-Containing Tailings with Metallurgical Slag-Based Binders. Materials 2022, 15, 1780. https://doi.org/10.3390/ma15051780
Li Y, Ni W, Gao W, Zhang S, Fu P, Li Y. Study on Solidification and Stabilization of Antimony-Containing Tailings with Metallurgical Slag-Based Binders. Materials. 2022; 15(5):1780. https://doi.org/10.3390/ma15051780
Chicago/Turabian StyleLi, Yunyun, Wen Ni, Wei Gao, Siqi Zhang, Pingfeng Fu, and Yue Li. 2022. "Study on Solidification and Stabilization of Antimony-Containing Tailings with Metallurgical Slag-Based Binders" Materials 15, no. 5: 1780. https://doi.org/10.3390/ma15051780