Phyllanthus emblica Seed-Derived Hierarchically Porous Carbon Materials for High-Performance Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. KOH Activation of Phyllanthus emblica Seed Powder
2.2. Characterizations of Phyllanthus emblica Seed-Derived Carbon Materials
2.3. Electrochemical Supercapacitance Performance Studies
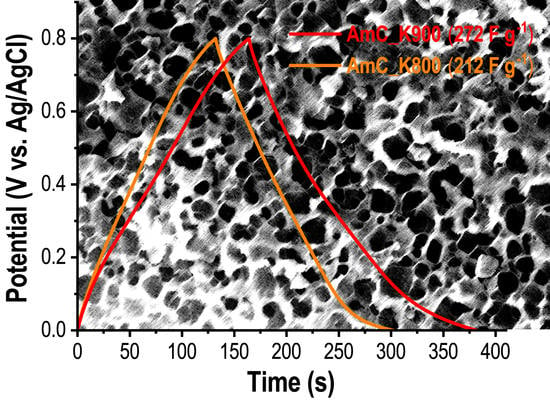
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, B.; Cao, L.; Yuan, Q.; Ma, Z.; Huang, Z.; Lin, Z.; Zhang, P. Biomass Straw-Derived Porous Carbon Synthesized for Supercapacitor for Ball Milling. Materials 2022, 15, 924. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xiang, R.; Lin, J.; Cheng, Y.; Lu, C. Lignocellulosic Biomass-Derived Carbon Electrode for Flexible Supercapacitors: An Overview. Materials 2021, 14, 4571. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Qin, C.; Wang, J.; Cao, L.; Ma, Z.; Yuan, Q.; Lin, Z.; Zhang, P. Research on High-Value Utilization of Carbon Derived Tobacco Waste in Supercapacitors. Materials 2021, 14, 1714. [Google Scholar] [CrossRef] [PubMed]
- Ariharan, A.; Kim, S.-K. Three-Dimensional Hierarchical Porous Carbon Derived from Betelnut Shells for Supercapacitor Electrodes. Materials 2021, 14, 7793. [Google Scholar] [CrossRef]
- Kigozi, M.; Kali, R.; Bello, A.; Padya, B.; Kalu-Uka, G.M.; Wasswa, J.; Jain, P.K.; Onwualu, P.A.; Dzade, N.Y. Modified Activation Process for Supercapacitor Electrode Materials from African Maize Cob. Materials 2020, 13, 5412. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Jakubec, P.; Bartusek, S.; Dvořáček, J.J.; Šedajová, V.; Kupak, V.; Otyepka, M. Flax-Derived Carbon: A Highly Durable Electrode Material for Electrochemical Double-Layer Supercapacitors. Nanomaterials 2021, 11, 2229. [Google Scholar] [CrossRef]
- Wang, T.; Hu, S.; Wu, D.; Zhao, W.; Yu, W.; Wang, M.; Xu, J.; Zhang, J. Boosting the Capacity of Biomass-Based Supercapacitors using Carbon Materials of Wood Derivatives and Redox Molecules from Plant. J. Mater. Chem. A 2021, 9, 11839–11852. [Google Scholar] [CrossRef]
- Huang, T.-F.; Hsieh, T.-H.; Tseng, F.-S.; Wang, L.-Y.; Yang, C.-C.; Yang, C.-C. High-Mass Loading Hierarchically Porous Activated Carbon Electrode for Pouch-Type Supercapacitors with Propylene Carbonate-Based Electrolyte. Nanomaterials 2021, 11, 786. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Thakur, A.K.; Adhikari, A.D.; Zhu, Y.; Wang, N. Current Research of Graphene-Based Nanocomposites and Their Application for Supercapacitors. Nanomaterials 2020, 10, 2046. [Google Scholar] [CrossRef]
- Shrestha, R.G.; Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications. Nanomaterials 2020, 10, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Nagaraju, G.; Panagiotopoulos, A.; Och, M.; Cheng, G.; Iacoviello, F.; Mattevi, C. Aqueous Inks of Pristine Graphene for 3D Printed Microsupercapacitors with High Capacitance. ACS Nano 2021, 15, 15342–15353. [Google Scholar] [CrossRef]
- Mao, W.; Yue, W.; Xu, Z.; Chang, S.; Hu, Q.; Pei, F.; Huang, Z.; Zhang, J.; Li, D.; Liu, G.; et al. Development of a Synergistic Activation Strategy for the Pilot-Scale Construction of Hierarchical Porous Graphitic Carbon for Energy Storage Applications. ACS Nano 2020, 14, 4741–4754. [Google Scholar] [CrossRef]
- Singh, G.; Bahadur, R.; Ruban, A.M.; Davidraj, J.M.; Su, D.; Vinu, A. Synthesis of Functionalized Nanoporous Biocarbons with High Surface Area for CO2 Capture and Supercapacitor Applications. Green Chem. 2021, 23, 5571–5583. [Google Scholar] [CrossRef]
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Sing, G.; Gopalan, A.-I.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.H.; Yi, J.; et al. Recent Advances in Functionalized Nanoporous Carbons Derived from Waste Resources and Their Applications in Energy and Environment. Adv. Sustain. Syst. 2021, 5, 2000169. [Google Scholar] [CrossRef]
- Sahoo, S.; Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Manoharan, S.; Kim, S.-J. High Performance Self-Charging Supercapacitors using a Porous PVDF-Ionic Liquid Electrolyte Sandwiched between Two-Dimensional Graphene Electrodes. J. Mater. Chem. A 2019, 7, 21693–21703. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A Review on the Recent Advances in Hybrid Supercapacitor. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Aken, K.L.V.; Deidaghi, M.; Gogotsi, Y. Formulation of Ionic-Liquid Electrolyte to Expand the Voltage Window of Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef]
- Pan, S.; Yao, M.; Zhang, J.; Li, B.; Xing, C.; Song, X.; Su, P.; Zhang, H. Recognition of Ionic Liquids as High-Voltage Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 261. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous Carbon Tubes from Fullerene Crystals as the π-Electron Carbon Source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Hill, J.P.; Tsuruoka, T.; Ji, Q.; Nishimura, T.; Ariga, K. Surfactant-Triggered Nanoarchitectonics of Fullerene C60 Crystals at a Liquid–Liquid Interface. Langmuir 2016, 32, 12511–12519. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous Carbon Cubes Derivied from Fullerene Crystals as a High Rate Performance Electrode Materials for Sueprcapacitors. J. Mater. Chem. A 2019, 7, 12654–12660. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Ilbeygi, H.; Park, D.-H.; Sarkar, S.; Chandra, G.; Umapathy, S.; Srinivasan, S.; Talapaneni, S.; Vinu, A. Highly Crystalline Mesoporous C60 with Ordered Pores: A Class of Nanomaterials for Energy Applications. Angew. Chem. Int. Ed. 2018, 57, 569–573. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Baskar, A.V.; Park, D.-H.; Chandra, G.; Umapathy, S.; Talapaneni, N.; Vinu, A. Ordered Mesoporous C70 with Highly Crystalline Pore Walls for Energy Applications. Adv. Funct. Mater. 2018, 28, 1803701. [Google Scholar] [CrossRef]
- Lu, Z.; Raad, R.; Safaei, F.; Xi, J.; Liu, Z.; Foroughi, J. Carbon Nanotube Based Fiber Supercapacitor as Wearable Energy Storage. Front. Mater. 2019, 6, 138. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Shrestha, R.G.; Subramani, T.; Maji, S.; Kim, J.H.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Indium Oxide/Carbon Nanotube/Reduced Graphene Oxide Ternary Nanocomposite with Enhanced Electrochemical Supercapacitance. Bull. Chem. Soc. Jpn. 2019, 92, 521–528. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Kim, J.; Tang, J.; Na, J.; Kang, Y.-M.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-Scale Synthesis of MOF-Derived Superporous Carbon Aerogels with Extraordinary Adsorption Capacity for Organic Solvents. Angew. Chem. Int. Ed. 2020, 59, 2066–2070. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New Strategies for Novel MOF-Derived Carbon Materials Based on Nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zheng, X.; Luo, J.; Kang, F.; Cheng, H.-M.; Yang, Q.-H. Ultra-Thick Graphene Bulk Supercapacitor Electrode for Compact Energy Storage. Energy Environ. Sci. 2016, 9, 3135–3142. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically Porous Carbon Derived from Polymers and Biomass: Effect of Interconnected Pores on Energy Applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent Advances in Functionalized Micro and Mesoporous Carbon Materials: Synthesis and Applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Su, F.; Xie, L.; Guo, X.; Wang, Z.; Kong, Q.; Sun, G.; Ahmad, A.; Li, X.; Yi, Z.; et al. Effect of Pore Structure and Doping Species on Charge Storage Mechanisms in Porous Carbon-Based Supercapacitors. Mater. Chem. Front. 2020, 4, 2610–2634. [Google Scholar] [CrossRef]
- Lyu, L.; Seong, K.-D.; Ko, D.; Choi, J.; Lee, C.; Hwang, T.; Cho, Y.; Jin, X.; Zhang, W.; Pang, H.; et al. Recent Development of Biomass-Derived Carbons and Composites as Electrode Materials for Supercapacitors. Mater. Chem. Front. 2019, 3, 2543–2570. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, K.; Wei, L.; Zhang, X.; Guo, X. Hierarchical Porous Microspheres of Activated Carbon with a High Surface Area from Spores for Electrochemical Double-Layer Capacitors. J. Mater. Chem. A 2016, 4, 15968–15979. [Google Scholar] [CrossRef]
- Karamanova, B.; Stoyanova, A.; Shipochka, M.; Veleva, S.; Stoyanova, R. Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials. Materials 2020, 13, 2941. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, M.; Nadria, M.P.; Sathish, M. Fabrication of Flexible Supercapacitor Using N-Doped Porous Activated Carbon Derived from Poultry Waste. Energy Fuels 2021, 18, 15094–15100. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-Derived Carbon Electrode Materials for Supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-Doped Mesoporous Carbon of Extraordinary Capacitance for Electrochemical Energy Storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef]
- Shrestha, R.G.; Maji, S.; Mallick, A.K.; Jha, A.; Shrestha, R.M.; Rajbhandari, R.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Hierarchically Porous Carbon from Phoenix dactylifera Seed for High-Performance Supercapacitor Applications. Bull. Chem. Soc. Jpn. 2022, 95, 1060–1067. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-Derived Porous Graphitic Carbon Materials for Energy and Environmental Applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Lu, T.; Xu, X.; Zhang, S.; Pan, L.; Wang, Y.; Alshehri, S.M.; Ahamad, T.; Kim, M.; Na, J.; Hossain, M.S.A.; et al. High-Performance Capacitive Deionization by Lignocellulose-Derived Eco-Friendly Porous Carbon Materials. Bull. Chem. Soc. Jpn. 2020, 93, 1014–1019. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Liu, Y.; Fan, L.-Z. Biowaste-Derived 3D Honeycomb-Like Porous Carbon with Binary-Heteroatom Doping for High-Performance Flexible Solid-State Supercapacitors. J. Mater. Chem. A 2018, 6, 160–166. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, Q.; Huo, B.; Zhou, H.; Huang, Z.; Li, H.; Yan, Z.; Yang, X.; Kuang, Y. Facile Self-Templating Synthesis of Heteroatom-Doped 3D Porous Carbon Materials from Waste Biomass for Supercapacitors. Chem. Commun. 2020, 56, 11689–11692. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Jiang, S.; Zhao, J.; Zhang, S.; Wang, R.; Li, N.; Liu, R.; Pan, Q.; Qu, W.; Xing, B. “Water-in-Salt” Electrolyte Enhanced High Voltage Aqueous Supercapacitor with Carbon Electrodes Derived from Biomass Waste-Ground Grain Hulls. RSC Adv. 2020, 10, 35545–35556. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Chaudhary, R.; Shrestha, R.G.; Shrestha, T.; Maji, S.; Ariga, K.; Shrestha, L.K. Washnut Seed-Derived Ultrahigh Surface Area Nanoporous Carbons as High Rate Performance Electrode Material for Supercapacitors. Bull. Chem. Soc. Jpn. 2021, 94, 565–572. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Choma, J.; Jaroniec, M. Recent Advances in the Development and Applications of Biomass-Derived Carbons with Uniform Porosity. J. Mater. Chem. A 2020, 8, 18464–18491. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Adhikari, L.; Shrestha, R.G.; Adhikari, M.P.; Adhikari, R.; Hill, J.P.; Pradhananga, R.R.; Ariga, K. Nanoporous Carbon Materials with Enhanced Supercapacitor Performance and Non-Aromatic Chemical Sensing with C1/C2 Alcohol Discrimination. Sci. Technol. Adv. Mater. 2016, 17, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Karnan, M.; Raj, A.G.K.; Subramani, K.; Santoshkumar, S.; Sathish, M. The Fascinating Supercapacitive Performance of Activated Carbon Electrodes with Enhanced Energy Density in Multifarious Electrolytes. Sustain. Energy Fuels 2020, 4, 3029–3041. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Chaudhary, R.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Nanoarchitectonics of Lotus Seed Derived Nanoporous Carbon Materials for Supercapacitor Applications. Materials 2020, 13, 5434. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Chaudhary, R.; Pradhananga, R.R.; Tamrakar, B.M.; Shrestha, T.; Maji, S.; Shretha, R.L.; Ariga, K. Nelumbo nucifera Seed-Derived Nitrogen-Doped Hierarchically Porous Carbons as Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2021, 11, 3175. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Maji, S.; Pokharel, B.P.; Rajbhandari, R.; Shrestha, R.L.; Pradhananga, R.; Hill, J.P.; Ariga, K. High Surface Area Nanoporous Graphitic Carbon Materials Derived from Lapsi Seed with Enhanced Supercapacitance. Nanomaterials 2020, 10, 728. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Gao, J.; Zhai, X.; Xie, M.; Sun, Y.; Kang, H.; Tian, Q.; Qiu, H. Hierarchical Porous Carbon Microrods Derived from Albizia Flower for High Performance Supercapacitors. Carbon 2019, 147, 242–251. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-Dimensional Honeycomb-Like Porous Carbon with Both Interconnected Hierarchical Porosity and Nitrogen Self-Doping from Cotton Seed Husk for Supercapacitor Electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A new analysis for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Golden, T.C.; Sircar, S. Activated Carbon Adsorbent for PSA Driers. Carbon 1990, 28, 683–690. [Google Scholar] [CrossRef]
- Schneider, P.; Hudec, P.; Solcova, O. Pore-Volume and Surface Area in Microporous–Mesoporous Solids. Microporous Mesoporous Mater. 2008, 115, 491–496. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Singh, C.; Cherian, I.; Giri, A.; Paul, A. Diciphering the Incredible Supercapacitor Performance Conducting Biordered Ultramicroporous Graphitic Carbon. ACS Appl. Energy Mater. 2021, 4, 4416–4427. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe Vera Derived Activated High-Surface-Area Carbon for Flexible and High-Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, N.; Simon, P.; Gogotsi, Y.; Presser, V. Increase in Capacitance by Subnanometer Pores in Carbon. ACS Energy Lett. 2016, 1, 1262–1265. [Google Scholar] [CrossRef] [Green Version]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient Storage Mechanism for Building Better Supercapacitors. Nature Energy 2016, 1, 16070. [Google Scholar] [CrossRef] [Green Version]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical Porous Carbon from Mango Seed Husk for Electro-Chemical Energy Storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W. A Biomass-Derived Nitrogen-Doped Porous Carbon for High-Energy Supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Liu, X.; Mi, R.; Yuan, L.; Yang, F.; Fu, Z.; Wang, C.; Tang, Y. Nitrogen-Doped Multi-Scale Porous Carbo for High Voltage Aqueous Supercapacitors. Fornt. Chem. 2018, 6, 475. [Google Scholar]
- Yoo, H.D.; Jang, J.H.; Ryu, J.H.; Park, Y.; Oh, S.M. Impedance Analysis of Porous Carbon Electrodes to Predict Rate Capability of Electric Double-Layer Capacitors. J. Power Sources 2014, 267, 411–420. [Google Scholar] [CrossRef]
- Abouelamaiem, D.I.; He, G.; Neville, T.P.; Patel, D.; Ji, S.; Wang, R.; Parkin, I.P.; Jorge, A.B.; Titirici, M.-M.; Shearing, P.R.; et al. Correlating Electrochemical Impedance with Hierarchical Structure for Porous Carbon-Based Supercapacitors using a Truncated Transmission Line Model. Electrochim. Acta 2018, 284, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Zhang, R.; Ren, Y. Naturally O-N-S Co-Doped Carbon with Multiscale Pore Architecture Derived from Lotus Leaf Stem for High-Performance Supercapacitors. Bull. Chem. Soc. Jpn. 2021, 94, 1705–1714. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Ariga, K.; Shrestha, L.K. Nanoporous Carbon Materials Derived from Washnut Seed with Enhanced Supercapacitance. Materials 2020, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Maji, S.; Shrestha, R.G.; Shrestha, R.L.; Shrestha, T.; Ariga, K.; Shrestha, L.K. Jackfruit Seed-Derived Nanoporous Carbon as the Electrode Material for Supercapacitors. C J. Carbon Res. 2020, 6, 73. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Ren, M.; Ge, Y.; Chen, H.; Ma, X.; Hao, Q. Preparation and Characterization of Porous Carbons by Pyrolysis-CO2 Gasification of Pine Sawdust. Chem. Lett. 2020, 49, 652–655. [Google Scholar] [CrossRef]
- Gehrke, V.; Maron, G.K.; Rodriguez, L.D.S.; Alano, J.H.; Pereira, C.M.P.D.; Orlandi, M.O.; Carreño, N.L.V. Facile Preparation of a Novel Biomass-Derived H3PO4 and Mn(NO3)2 Activated Carbon from citrus bergamia Peels for High-Performance Supercapacitors. Mater. Today Commun. 2021, 26, 101779. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Muthusamy, A.; Cho, I.; Kim, H.-J.; Senthil, K.; Prabakar, K. Ultrahigh Surface Area Biomass Derived 3D Hierarchical Porous Carbon Nanosheet Electrodes for High Energy Density Supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Song, Y.; Qu, W.W.; He, Y.H.; Yang, H.X.; Du, M.; Wang, A.J.; Yang, Q.; Chen, Y.Q. Synthesis and Processing Optimization of N-doped Hierarchical Porous Carbon Derived from Corncob for High Performance Supercapacitors. J. Energy Storage 2020, 32, 101877. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Gao, Y.; An, W.; Cao, Z.; Liu, J. Biomass-Swelling Assisted Synthesis of Hierarchical porous Carbon Fibers for Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28283–28290. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Zhang, Y.; Zhou, X.; Wang, L.; Yasin, A.; Zhang, L. Bioresource Derived Porous Carbon from Cottonseed Hull for Removal of Triclosan and Electrochemical Application. RSS Adv. 2018, 8, 42405–42414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Wang, Q.; Chang, W.; Huan, S.; Hu, Y.; Niu, Z.; Hang, G.; Cheng, H.; Wang, G. A Novel Strategy Combining Electrospraying and One-step Carbonization for the Preparation of Ultralight Honeycomb-like Multilayered Carbon from Biomass-derived Lignin. Carbon 2021, 179, 68–79. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Ladmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M. -M. From Waste to Wealth: From Kraft Lignin to Free-standing Supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Chen, H.; Liu, Y.; Yang, D.; Li, H. Graphene-like Porous Carbon Nanosheets Derived from Salvia Splendens for High-rate Performance Supercapacitors. J. Power Sources 2018, 397, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, J.; Dong, S.; Zhang, Y.; An, Y.; Ding, B.; Zhang, T.; Dou, H.; Zhang, X.J. Biomass-derived Porous Carbon Electrodes for High-performance Supercapacitors. J. Mater. Sci. 2020, 55, 5166–5176. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, L.; Liu, Y.; Zhao, G.; Chen, J.Y.; Yu, G. Biobased Nanoporous Active Carbon Fibers for High-performance Supercapacitors. ACS Appl. Mater. Interface 2016, 8, 15205–15215. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, H.; Hu, Y.; Lai, H.; Liu, L.; Zhong, L.; Peng, X. Wood-derived Lightweight and Elastic Carbon Aerogel for Pressure Sensing and Energy Storage. Adv. Funct. Mater. 2020, 30, 1910292. [Google Scholar] [CrossRef]








| Sample | SSA (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Vp (cm3 g−1) | Vmicro (cm3 g−1) | Wp (nm) | Dp (nm) |
|---|---|---|---|---|---|---|---|
| AmP_900 | 109 | 43 | 66 | 0.231 | 0.101 | - | 3.09 |
| AmC_K700 | 1360 | 1268 | 92 | 0.664 | 0.491 | 0.286 | 3.09 |
| AmC_K800 | 1779 | 1707 | 72 | 0.923 | 0.794 | 0.299 | 3.09 |
| AmC_K900 | 1946 | 1799 | 147 | 1.115 | 0.885 | 0.286 | 3.47 |
| AmC_K1000 | 1099 | 770 | 329 | 1.328 | 0.763 | 0.705 | 3.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, L.K.; Shahi, S.; Gnawali, C.L.; Adhikari, M.P.; Rajbhandari, R.; Pokharel, B.P.; Ma, R.; Shrestha, R.G.; Ariga, K. Phyllanthus emblica Seed-Derived Hierarchically Porous Carbon Materials for High-Performance Supercapacitor Applications. Materials 2022, 15, 8335. https://doi.org/10.3390/ma15238335
Shrestha LK, Shahi S, Gnawali CL, Adhikari MP, Rajbhandari R, Pokharel BP, Ma R, Shrestha RG, Ariga K. Phyllanthus emblica Seed-Derived Hierarchically Porous Carbon Materials for High-Performance Supercapacitor Applications. Materials. 2022; 15(23):8335. https://doi.org/10.3390/ma15238335
Chicago/Turabian StyleShrestha, Lok Kumar, Sabina Shahi, Chhabi Lal Gnawali, Mandira Pradhananga Adhikari, Rinita Rajbhandari, Bhadra P. Pokharel, Renzhi Ma, Rekha Goswami Shrestha, and Katsuhiko Ariga. 2022. "Phyllanthus emblica Seed-Derived Hierarchically Porous Carbon Materials for High-Performance Supercapacitor Applications" Materials 15, no. 23: 8335. https://doi.org/10.3390/ma15238335