Green Synthesis of Molecularly Imprinted Polymers for Dispersive Magnetic Solid-Phase Extraction of Erythrosine B Associated with Smartphone Detection in Food Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Image Processing
2.3. Preparation of Magnetic Nanoparticles
2.4. Synthesis of MIPs for ERT-B
2.5. Binding Experiments
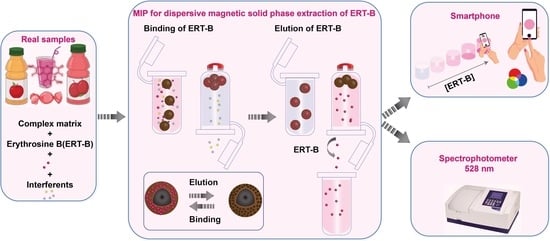
2.6. MIP-Magnetic Dispersive Solid-Phase Extraction (MIP-MDSPE)
2.7. Determination of ERT-B in Food Samples
3. Results and Discussion
3.1. MIP-Dispersive Solid-Phase Extraction Optimization
3.2. MIPs Performance
3.2.1. Isotherm Modelling
3.2.2. Adsorption Kinetics
3.2.3. FT-IR Characterization
3.2.4. Reusability
3.3. Analytical Determination of ERT-B
3.3.1. Photometric Detection
3.3.2. Smartphone Detection
3.4. Selectivity Study
3.5. Preconcentration
3.6. Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pipíška, M.; Krajčíková, E.K.; Hvostik, M.; Frišták, V.; Ďuriška, L.; Černičková, I.; Kaňuchová, M.; Conte, P.; Soja, G. Biochar from Wood Chips and Corn Cobs for Adsorption of Thioflavin T and Erythrosine B. Materials 2022, 15, 1492. [Google Scholar] [CrossRef] [PubMed]
- Bistaffa, M.J.; Camacho, S.A.; Melo, C.F.O.R.; Catharino, R.R.; Toledo, K.A.; Aoki, P.H.B. Plasma membrane permeabilization to explain erythrosine B phototoxicity on in vitro breast cancer cell models. J. Photochem. Photobiol. B Biol. 2021, 223, 112297. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T. Toxicity of xanthene food dyes by inhibition of human drug-metabolizing enzymes in a noncompetitive manner. J. Environ. Public Health 2009, 2009, 953952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chequer, F.M.D.; Venâncio, V.D.P.; Bianchi, M.D.L.P.; Antunes, L.M.G. Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells. Food Chem. Toxicol. 2012, 50, 3447–3451. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Barrows, J.; Bechaux, C.; Benford, D.; Cantril, R.; DiNovi, M. Erythrosine (Consolidated Monograph); WHO Food Additives Series, No. 77; WHO: Geneva, Switzerland, 2020; p. 105. [Google Scholar]
- Zeyada, H.M.; El-Mallah, H.M.; Atwee, T.; El-Damhogi, D.G. Spectroscopic studies of UV irradiated erythrosine B thin films prepared by spin coating technique. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2017, 179, 120–124. [Google Scholar] [CrossRef]
- Pinilla-Peñalver, E.; Villaseñor, M.J.; Contento, A.M.; Ríos, Á. Erythrosine B—Coated gold nanoparticles as an analytical sensing tool for the proper determination of both compounds based on surface-enhanced Raman spectroscopy. Microchem. J. 2020, 157, 104937. [Google Scholar] [CrossRef]
- Ma, K.; Song, Z.L.; Chao, J.B.; Wei, C.; Zhang, J.N. Determination of trace sodium iodide in food color additive erythrosine using high performance liquid chromatography with inductively coupled plasma-mass spectrometer. Jiliang Xuebao/Acta Metrol. Sin. 2012, 33, 368–371. [Google Scholar] [CrossRef]
- Nayak, D.S.; Shetti, N.P. A novel sensor for a food dye erythrosine at glucose modified electrode. Sens. Actuators B Chem. 2016, 230, 140–148. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, W.; Wang, Y.; Zhu, L.; Yang, L.; Sha, Z.; Zhang, J. Decoration of graphene with 2-aminoethanethiol functionalized gold nanoparticles for molecular imprinted sensing of erythrosine. Carbon 2018, 127, 618–626. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Kuchinad, G.T. Electrochemical oxidation of erythrosine at TiO2 nanoparticles modified gold electrode—An environmental application. J. Environ. Chem. Eng. 2017, 5, 2083–2089. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Jones, G.; Stringer, F.; Wilson, I. Comparison of extraction of a β-blocker from plasma onto a molecularly imprinted polymer with liquid–liquid extraction and solid phase extraction methods. J. Pharm. Biomed. Anal. 2004, 35, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mittal, A.; Kurup, L.; Mittal, J. Adsorption of a hazardous dye, erythrosine, over hen feathers. J. Colloid Interface Sci. 2006, 304, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Carmen Apostol, L.; Ghinea, C.; Alves, M.; Gavrilescu, M. Removal of Erythrosine B dye from water effluents using crop waste pumpkin seed hulls as adsorbent. Desalination Water Treat. 2016, 57, 22585–22608. [Google Scholar] [CrossRef] [Green Version]
- Çetinkaya, H.F.; Cebeci, M.S.; Kaya, S.; Jalbani, N.S.; Maslov, M.M.; Marzouki, R. Removal of erythrosine B dye from wastewater using chitosan boric acid composite material: Experimental and density functional theory findings. J. Phys. Org. Chem. 2022, e4400. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Adsorptive removal of Erythrosine dye onto activated low cost de-oiled mustard. J. Hazard. Mater. 2009, 164, 627–633. [Google Scholar] [CrossRef]
- Elfadil, D.; Lamaoui, A.; Della Pelle, F.; Amine, A.; Compagnone, D. Molecularly imprinted polymers combined with electrochemical sensors for food contaminants analysis. Molecules 2021, 26, 4607. [Google Scholar] [CrossRef]
- Elfadil, D.; Palmieri, S.; Della Pelle, F.; Sergi, M.; Amine, A.; Compagnone, D. Enzyme inhibition coupled to molecularly imprinted polymers for acetazolamide determination in biological samples. Talanta 2022, 240, 123195. [Google Scholar] [CrossRef]
- Elfadil, D.; Palmieri, S.; Silveri, F.; Della Pelle, F.; Sergi, M.; Del Carlo, M.; Amine, A.; Compagnone, D. Fast sonochemical molecularly imprinted polymer synthesis for selective electrochemical determination of maleic hydrazide. Microchem. J. 2022, 180, 107634. [Google Scholar] [CrossRef]
- Lamaoui, A.; Cubillana-Aguilera, L.; Gil, M.L.A.; Amine, A.; Palacios-Santander, J.M. Chapter 16 Analytical Applications of Molecularly Imprinted Polymer-decorated Magnetic Nanoparticles. In Analytical Applications of Functionalized Magnetic Nanoparticles; The Royal Society of Chemistry: London, UK, 2021; pp. 397–428. [Google Scholar]
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Molecularly imprinted polymers based on polydopamine: Assessment of non-specific adsorption. Microchem. J. 2021, 164, 106043. [Google Scholar] [CrossRef]
- Scroccarello, A.; Della Pelle, F.; Ferraro, G.; Fratini, E.; Tempera, F.; Dainese, E.; Compagnone, D. Plasmonic active film integrating gold/silver nanostructures for H2O2 readout. Talanta 2021, 222, 121682. [Google Scholar] [CrossRef]
- Scroccarello, A.; Della Pelle, F.; Fratini, E.; Ferraro, G.; Scarano, S.; Palladino, P.; Compagnone, D. Colorimetric determination of polyphenols via a gold nanoseeds–decorated polydopamine film. Microchim. Acta 2020, 187, 267. [Google Scholar] [CrossRef] [PubMed]
- Scroccarello, A.; Della Pelle, F.; Del Carlo, M.; Compagnone, D. Monitoring disinfection in the Covid-19 era. A reagent-free nanostructured smartphone-based device for the detection of oxidative disinfectants. Microchem. J. 2022, 175, 107165. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Della Pelle, F.; Mascini, M.; Cichelli, A.; Compagnone, D. Peptides, DNA and MIPs in gas sensing. From the realization of the sensors to sample analysis. Sensors 2020, 20, 4433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Tang, D.; Zhang, Y.; Xu, L.; Liu, K.; Huang, K.; Yin, Z. Specific and Sensitive Detection of Tartrazine on the Electrochemical Interface of a Molecularly Imprinted Polydopamine-Coated PtCo Nanoalloy on Graphene Oxide. Biosensors 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shao, J.; Sui, G.; Ma, Y.; Li, H. Rapid detection of orange II dyes in water with SERS imprinted sensor based on PDA-modified MOFs@Ag. J. Environ. Chem. Eng. 2021, 9, 106317. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Daneshfar, A.; Darafarin, S.; Sahraei, R.; Purkait, M. Simultaneous ultrasound-assisted removal of sunset yellow and erythrosine by ZnS: Ni nanoparticles loaded on activated carbon: Optimization by central composite design. Ultrason. Sonochemistry 2014, 21, 1441–1450. [Google Scholar] [CrossRef]
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Fast microwave-assisted synthesis of magnetic molecularly imprinted polymer for sulfamethoxazole. Talanta 2021, 232, 122430. [Google Scholar] [CrossRef]
- Ahmad, Z.; Li, Y.; Ali, S.; Yang, J.; Jan, F.; Fan, Y.; Gou, X.; Sun, Q.; Chen, J. Benignly-fabricated supramolecular poly(amidoxime)-alginate-poly(acrylic acid) beads synergistically enhance uranyl capture from seawater. Chem. Eng. J. 2022, 441, 136076. [Google Scholar] [CrossRef]
- Ahmad, Z.; Li, Y.; Yang, J.; Geng, N.; Fan, Y.; Gou, X.; Sun, Q.; Chen, J. A Membrane-Supported Bifunctional Poly(amidoxime-ethyleneimine) Network for Enhanced Uranium Extraction from Seawater and Wastewater. J. Hazard. Mater. 2022, 425, 127995. [Google Scholar] [CrossRef]
- Karrat, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. A novel magnetic molecularly imprinted polymer for selective extraction and determination of quercetin in plant samples. Anal. Chim. Acta 2022, 1203, 339709. [Google Scholar] [CrossRef] [PubMed]
- Karrat, A.; Amine, A. Solid-phase extraction combined with a spectrophotometric method for determination of Bisphenol-A in water samples using magnetic molecularly imprinted polymer. Microchem. J. 2021, 168, 106496. [Google Scholar] [CrossRef]
- Kaur, M.; Datta, M. Adsorption equilibrium and kinetics of toxic dye-Erythrosine B adsorption onto montmorillonite. Sep. Sci. Technol. 2013, 48, 1370–1381. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Rojanarata, T.; Ngawhirunpat, T.; Akkaramongkolporn, P.; Kaomongkolgit, R.; Opanasopit, P. Erythrosine Incorporated Fast-Dissolving Patches for Dental Plaque Disclosing. Adv. Pharmacol. Pharm. 2017, 5, 12–19. [Google Scholar] [CrossRef]
- Sharifzade, G.; Asghari, A.; Rajabi, M. Highly effective adsorption of xanthene dyes (rhodamine B and erythrosine B) from aqueous solutions onto lemon citrus peel active carbon: Characterization, resolving analysis, optimization and mechanistic studies. RSC Adv. 2017, 7, 5362–5371. [Google Scholar] [CrossRef] [Green Version]
- Prasada Rao, T.; Daniel, S.; Mary Gladis, J. Tailored materials for preconcentration or separation of metals by ion-imprinted polymers for solid-phase extraction (IIP-SPE). Trends Anal. Chem. 2004, 23, 28–35. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, L.; Yin, Y.; Wang, J.; Wang, P.; Ren, L.; Eremin, S.A.; He, X.; Meng, M.; Xi, R. A sensitive competitive enzyme immunoassay for detection of erythrosine in foodstuffs. Food Control 2015, 47, 472–477. [Google Scholar] [CrossRef]
- Chou, S.-S.; Lin, Y.-H.; Cheng, C.-C.; Hwang, D.-F. Determination of Synthetic Colors in Soft Drinks and Confectioneries by Micellar Electrokinetic Capillary Chromatography. J. Food Sci. 2002, 67, 1314–1318. [Google Scholar] [CrossRef]
- Berzas Nevado, J.J.; Guiberteau Cabanillas, C.; Contento Salcedo, A.M. Method development and validation for the simultaneous determination of dyes in foodstuffs by capillary zone electrophoresis. Anal. Chim. Acta 1999, 378, 63–71. [Google Scholar] [CrossRef]
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef]
- Palianskikh, A.I.; Sychik, S.I.; Leschev, S.M.; Pliashak, Y.M.; Fiodarava, T.A.; Belyshava, L.L. Development and validation of the HPLC-DAD method for the quantification of 16 synthetic dyes in various foods and the use of liquid anion exchange extraction for qualitative expression determination. Food Chem. 2022, 369, 130947. [Google Scholar] [CrossRef] [PubMed]








| Models | Parameters | MIP | NIP |
|---|---|---|---|
| Freundlich | R2 | 0.945 | 0.827 |
| KF (mg·g−1) | 2.434 | 0.615 | |
| nF | 2.794 | 2.488 | |
| Langmuir | R2 | 0.993 | 0.970 |
| Kl (L·mg−1) | 0.996 | 0.602 | |
| Qmax (mg·g−1) | 5.706 | 0.970 | |
| Pseudo-first order | R2 | 0.9498 | 0.921 |
| Qe (mg·g−1) | 2.538 | 0.238 | |
| K1 (min−1) | 0.219 | 0.238 | |
| Pseudo-second order | R2 | 0.938 | 0.911 |
| Qe (mg·g−1) | 2.911 | 0.217 | |
| K2 (g mg−1 min−1) | 0.098 | 0.911 |
| UV–Vis | Smartphone | |||||
|---|---|---|---|---|---|---|
| ERB Added | ERB Found | Recovery | ERB Added | ERB Found | Recovery | RSD |
| (mg/L) | (mg/L) | (%) | (mg/L) | (mg/L) | (%) | (%) |
| Juice | ||||||
| 0.5 | 0.4 ± 0.01 | 80 | 0.5 | 0.4 ± 0.01 | 96 | 4 |
| 1 | 0.9 ± 0.01 | 93 | 1 | 0.9 ± 0.02 | 93 | 2 |
| 2 | 1.6 ± 0.02 | 81 | 2 | 1.9 ± 0.08 | 95 | 4 |
| 5 | 4.4 ± 0.13 | 88 | 5 | 4.5 ± 0.02 | 90 | 5 |
| Candied cherries | ||||||
| 0.5 | 0.5 ± 0.01 | 93 | 0.5 | 0.4 ± 0.02 | 96 | 5 |
| 1 | 0.9 ± 0.04 | 92 | 1 | 0.9 ± 0.04 | 91 | 5 |
| 2 | 1.6 ± 0.02 | 81 | 2 | 1.8 ± 0.07 | 91 | 4 |
| 5 | 4.6 ± 0.18 | 92 | 5 | 4.1 ± 0.25 | 82 | 6 |
| Candy | ||||||
| 0.5 | 0.4 ± 0.01 | 80 | 0.5 | 0.4 ± 0.02 | 88 | 5 |
| 1 | 0.8 ± 0.02 | 80 | 1 | 0.9 ± 0.03 | 97 | 3 |
| 2 | 1.6 ± 0.03 | 81 | 2 | 1.9 ± 0.10 | 97 | 5 |
| 5 | 4.0 ± 0.08 | 84 | 5 | 4.4 ± 0.22 | 88 | 5 |
| Method | LOD (mg/L) | Real Sample | Recovery | Ref. |
|---|---|---|---|---|
| ELISA | 0.0022 | Healthy energy drink, Breezer, grape juice, Coca-Cola sugar, fermented bean curd and tomato paste | 86.3% to 115.5% | [39] |
| Micellar electrokinetic capillary chromatography | 0.01 | Soft drinks and confectioneries | 82% | [40] |
| Capillary zone electrophoresis | 0.35 | Bitter, grenadine, ice lolly, strawberry and raspberry | 111% to 95.00% | [41] |
| HPLC using a short column with photodiode array detection | 0.011 | Soft drinks and candies | 76.6 to 115.0% | [42] |
| HPLC-DAD method | 0.026 | Dry red wine, milkshake, fish, tomato and paste | 83.7 to 107.5% | [43] |
| Colorimetric reading via smartphone | 0.04 | Juice, candy, and candied cherries | 82% to 97% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfadil, D.; Della Pelle, F.; Compagnone, D.; Amine, A. Green Synthesis of Molecularly Imprinted Polymers for Dispersive Magnetic Solid-Phase Extraction of Erythrosine B Associated with Smartphone Detection in Food Samples. Materials 2022, 15, 7653. https://doi.org/10.3390/ma15217653
Elfadil D, Della Pelle F, Compagnone D, Amine A. Green Synthesis of Molecularly Imprinted Polymers for Dispersive Magnetic Solid-Phase Extraction of Erythrosine B Associated with Smartphone Detection in Food Samples. Materials. 2022; 15(21):7653. https://doi.org/10.3390/ma15217653
Chicago/Turabian StyleElfadil, Dounia, Flavio Della Pelle, Dario Compagnone, and Aziz Amine. 2022. "Green Synthesis of Molecularly Imprinted Polymers for Dispersive Magnetic Solid-Phase Extraction of Erythrosine B Associated with Smartphone Detection in Food Samples" Materials 15, no. 21: 7653. https://doi.org/10.3390/ma15217653