Mechanisms and Critical Technologies of Transport Inhibitor Agent (TIA) throughout C-S-H Nano-Channels
Abstract
:1. Introduction
2. Methods
2.1. Molecular Dynamics Methods
2.1.1. Models
2.1.2. Force Fields and Simulation Details
2.2. Experimental Methods
2.2.1. Materials
2.2.2. Experimental Testing
3. Results and Discussion
3.1. Percolation Kinetics of Erosion Solutions in C-S-H Gel Channels
3.2. Adsorption Behavior of TIA in C-S-H Gel Channels
3.3. Inhibitory Transport Kinetics of TIA in C-S-H Gel Channels
3.4. Durability Test Verification
4. Conclusions
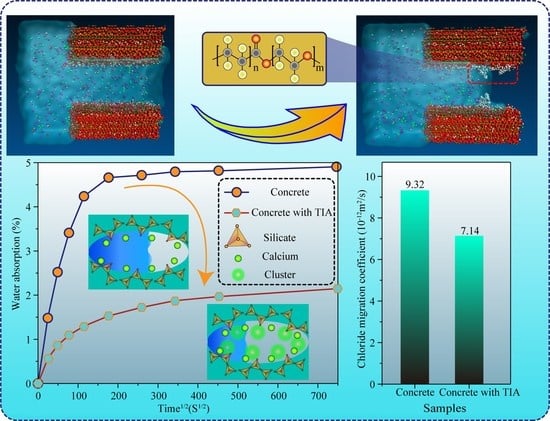
- Molecular dynamics unravel the interaction mechanisms between TIA and the C-S-H gel matrix. TIA has hydrophilic and hydrophobic groups, which are linked by carboxyl groups.
- The structure–activity relationship contributes to the design of TIA. Hydrophilic groups in TIA can effectively adsorb to the surface of the C-S-H matrix by bonding with calcium ions, while hydrophobic carbon chains on the other end can float on the solution surface through low-hydrogen bonding when the solution is in contact with it; thus, the effective diameter of the gel pore can be reduced, and the solution penetration can be prevented with the ‘door’ of the gel pore.
- In corrosive solution, Ca2+ in C-S-H chelates with TIA and corrosive ions, forming clusters to block gel pore channels, making the blocking effect more obvious. The experimental test results manifest that the water adsorption capacity, chloride ion migration rate, and chloride content of the concrete samples reduced significantly after adding TIA, indicating that TIA can effectively strengthen the durability of concrete.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, J.G.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Ferreira, R.M. Pobability-Based Durability Analysis of Concrete Structures in Marine Environment. Ph.D. Thesis, University of Minho, Guimarães, Portugal, November 2004. [Google Scholar]
- Maage, M.; Helland, S.; Poulsen, E.; Vennesland, Ø.; Carlsen, J.E. Service life prediction of existing concrete structures exposed to marine environment. ACI Mater. J. 1996, 93, 602–608. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Borba, J.C.; Padilha, M. Durability of concrete structures in marine atmosphere zones—The use of chloride deposition rate on the wet candle as an environmental indicator. Cem. Concr. Compos. 2010, 32, 427–435. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Padaratz, I.J.; Alonso, C.; Borba, J.C. Chloride penetration into concrete structures in the marine atmosphere zone—Relationship between deposition of chlorides on the wet candle and chlorides accumulated into concrete. Cem. Concr. Compos. 2007, 29, 667–676. [Google Scholar] [CrossRef]
- Page, C.L. Mechanism of corrosion protection in reinforced concrete marine structures. Nature 1975, 258, 514–515. [Google Scholar] [CrossRef]
- Akiyama, M.; Frangopol, D.M.; Suzuki, M. Integration of the effects of airborne chlorides into reliability-based durability design of reinforced concrete structures in a marine environment. Struct. Infrastruct. Eng. 2012, 8, 125–134. [Google Scholar] [CrossRef]
- Val, D.V.; Stewart, M.G. Life-cycle cost analysis of reinforced concrete structures in marine environments. Struct. Saf. 2003, 25, 343–362. [Google Scholar] [CrossRef]
- Pack, S.W.; Jung, M.S.; Song, H.W.; Kim, S.H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Taylor, F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Jennings, H.M.; Bullard, J.W. From electrons to infrastructure: Engineering concrete from the bottom up. Cem. Concr. Res. 2011, 41, 727–735. [Google Scholar] [CrossRef]
- Hou, D.; Ma, H.; Li, Z. Morphology of calcium silicate hydrate (C-S-H) gel: A molecular dynamic study. Adv. Cem. Res. 2015, 27, 135–146. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Li, W. Effect of PDMS on the waterproofing performance and corrosion resistance of cement mortar. Appl. Surf. Sci. 2020, 507, 145016. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The effect of silane-based hydrophobic admixture on corrosion of galvanized reinforcing steel in concrete. Corros. Sci. 2010, 52, 2958–2963. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Xu, W.; Liu, H.; Lu, Y. Inexpensive and non-fluorinated superhydrophobic concrete coating for anti-icing and anti-corrosion. J. Colloid Interface Sci. 2019, 541, 86–92. [Google Scholar] [CrossRef]
- Liu, J.; Cai, J.; Shi, L.; Liu, J.; Mu, S.; Hong, J. The inhibition behavior of a water-soluble silane for reinforcing steel in 3.5% NaCl saturated Ca(OH)2 solution. Constr. Build. Mater. 2018, 189, 95–101. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Hou, D.; Yu, J.; Zhao, T.; Yin, B. Experiment and molecular dynamics study on the mechanism for hydrophobic impregnation in cement-based materials: A case of octadecane carboxylic acid. Constr. Build. Mater. 2019, 229, 116871. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. Comparison between surface and bulk hydrophobic treatment against corrosion of galvanized reinforcing steel in concrete. Cem. Concr. Res. 2011, 41, 609–614. [Google Scholar] [CrossRef]
- Liu, J.; Mu, S.; Cai, J.; Jiang, Q. Preparation and performance evaluation of cement hydration responsive nanomaterial. J. Build. Struct. 2019, 40, 181–187. [Google Scholar] [CrossRef]
- Oltulu, M.; Şahin, R. Single and combined effects of nano-SiO2, nano-Al2O3 and nano-Fe2O3 powders on compressive strength and capillary permeability of cement mortar containing silica fume. Mater. Sci. Eng. A 2011, 528, 7012–7019. [Google Scholar] [CrossRef]
- Zhang, P.; Wan, J.; Wang, K.; Li, Q. Influence of nano-SiO2 on properties of fresh and hardened high performance concrete: A state-of-the-art review. Constr. Build. Mater. 2017, 148, 648–658. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, H.; Liu, J.; Guo, S.; Pei, Y. Improving the microstructure of ITZ and reducing the permeability of concrete with various water/cement ratios using nano-silica. J. Mater. Sci. 2019, 54, 444–456. [Google Scholar] [CrossRef]
- Marchon, D.; Juilland, P.; Gallucci, E.; Frunz, L.; Flatt, R.J. Molecular and submolecular scale effects of comb-copolymers on tri-calcium silicate reactivity: Toward molecular design. J. Am. Ceram. Soc. 2017, 100, 817–841. [Google Scholar] [CrossRef] [Green Version]
- Marchon, D.; Boscaro, F.; Flatt, R.J. First steps to the molecular structure optimization of polycarboxylate ether superplasticizers: Mastering fluidity and retardation. Cem. Concr. Res. 2019, 115, 116–123. [Google Scholar] [CrossRef]
- Marchon, D.; Mantellato, S.; Eberhardt, A.B.; Flatt, R.J. Adsorption of Chemical Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081006962. [Google Scholar]
- Dalas, F.; Nonat, A.; Pourchet, S.; Mosquet, M.; Rinaldi, D.; Sabio, S. Tailoring the anionic function and the side chains of comb-like superplasticizers to improve their adsorption. Cem. Concr. Res. 2015, 67, 21–30. [Google Scholar] [CrossRef]
- Pochard, I.; Labbez, C.; Nonat, A.; Vija, H.; Jönsson, B. The effect of polycations on early cement paste. Cem. Concr. Res. 2010, 40, 1488–1494. [Google Scholar] [CrossRef]
- Mailvaganam, N.P.; Rixom, M.R.; Manson, D.P.; Gonzales, C. Chemical Admixtures for Concrete; CRC Press: London, UK, 1999; ISBN 9780429215148. [Google Scholar]
- Allyn, M.; Frantz, G.C. Corrosion tests with concrete containing salts of alkenyl-substituted succinic acid. ACI Mater. J. 2001, 98, 224–232. [Google Scholar] [CrossRef]
- Zhong, S.; Tan, M.; Chen, Z. Chloride Diffusivity of Styrene-Acrylate Latex Modified Mortars. J. Build. Mater. 2002, 5, 6. [Google Scholar]
- Liu, G.; Liu, Y.; Cheng, D. Effect of Styrene Acrylic Emulsion on the Chloride Ion Penetration Property of Modified Concrete. In Proceedings of the 1st International Conference on Microstructure Related Durability of Cementitious Composites, Nanjing, China, 13–15 October 2008. [Google Scholar]
- Mindess, S.; Young, F.J.; Darwin, D. Concrete, 2nd ed.; Prentice Hall, Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Zhou, Y.; Zheng, H.; Li, W.; Ma, T.; Miao, C. A deep learning potential applied in tobermorite phases and extended to calcium silicate hydrates. Cem. Concr. Res. 2022, 152, 106685. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Liu, L.; She, W.; Yu, J. Microporous and Mesoporous Materials Experimental and molecular dynamics studies on the transport and adsorption of chloride ions in the nano-pores of calcium silicate phase: The in fluence of calcium to silicate ratios. Microporous Mesoporous Mater. 2018, 255, 23–35. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; She, W.; Li, J. Molecular dynamics study of solvated aniline and ethylene glycol monomers confined in calcium silicate nanochannels: A case study of tobermorite. Phys. Chem. Chem. Phys. 2017, 19, 15145–15159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; She, W.; Hou, D.; Yin, B.; Chang, H.; Jiang, J.; Li, J. Modification of incorporation and in-situ polymerization of aniline on the nano-structure and meso-structure of calcium silicate hydrates. Constr. Build. Mater. 2018, 182, 459–468. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; She, W.; Yu, J. Reactive molecular simulation on the calcium silicate hydrates/polyethylene glycol composites. Chem. Phys. Lett. 2017, 687, 184–187. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D. mechanisms of calcium-silicate-hydrate/polymer. Phys. Chem. Chem. Phys. 2018, 20, 8247–8266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, L.; Liu, J.; Miao, C. Interaction mechanisms between organic and inorganic phases in calcium silicate hydrates/poly(vinyl alcohol) composites. Cem. Concr. Res. 2019, 125. [Google Scholar] [CrossRef]
- Zhou, Y.; Orozco, C.A.; Duque-Redondo, E.; Manzano, H.; Geng, G.; Feng, P.; Monteiro, P.J.M.; Miao, C. Modification of poly(ethylene glycol) on the microstructure and mechanical properties of calcium silicate hydrates. Cem. Concr. Res. 2019, 115, 20–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Yang, X.; Dong, Y.; Feng, T.; Liu, J. Enhancing the PVA fiber-matrix interface properties in ultra high performance concrete: An experimental and molecular dynamics study. Constr. Build. Mater. 2021, 285, 122862. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.C.; Huang, J.L.; Ma, T.; Huang, X.M.; Miao, C.W. A molecular dynamics study of calcium silicate hydrates-aggregate interfacial interactions and influence of moisture. J. Cent. South Univ. 2021, 28, 16–28. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Manzano, H.; Orozco, C.A.; Geng, G.; Monteiro, P.J.M.; Liu, J. Interfacial Connection Mechanisms in Calcium-Silicate-Hydrates/Polymer Nanocomposites: A Molecular Dynamics Study. ACS Appl. Mater. Interfaces 2017, 9, 41014–41025. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Chen, R.; Hou, D.; Xu, J.; Lv, K.; Zheng, Q. The design and evaluation of a smart polymer-based fluids transport inhibitor. J. Clean. Prod. 2020, 257, 120528. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Hou, D.; Chang, H.; Yu, J. The inhibiting effect and mechanisms of smart polymers on the transport of fluids throughout nano-channels. Appl. Surf. Sci. 2020, 500, 144019. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Yang, Q.; Hou, D.; Hu, X.; Ye, Q.; Wang, D.; Ding, Q. Characterization of fly ash-cement paste and molecular structure in the presence of seawater by 27Al and 29Si MAS NMR spectroscopy. Constr. Build. Mater. 2020, 262, 120823. [Google Scholar] [CrossRef]
- Han, C.; Shen, W.; Ji, X.; Wang, Z.; Ding, Q.; Xu, G.; Lv, Z.; Tang, X. Behavior of high performance concrete pastes with different mineral admixtures in simulated seawater environment. Constr. Build. Mater. 2018, 187, 426–438. [Google Scholar] [CrossRef]
- Thomas, M. Chloride thresholds in marine concrete. Cem. Concr. Res. 1996, 26, 513–519. [Google Scholar] [CrossRef]
- Polder, R.B.; De Rooij, M.R. Durability of marine concrete structures—Field investigations and modelling. Heron 2005, 50, 133–154. [Google Scholar]
- Cerny, R.; Rovnaníková, P. Transport Processes in Concrete; CRC Press: London, UK, 2002; ISBN 1482289105. [Google Scholar]
- Shahsavari, R.; Pellenq, J.; Ulm, F. Empirical force fields for complex hydrated calcio-silicate layered materials. Phys. Chem. Chem. Phys. 2011, 13, 1002–1011. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhang, C.; Lu, X. A combined first principles and classical molecular dynamics study of clay-soil organic matters (SOMs) interactions. Geochim. Cosmochim. Acta 2020, 291, 110–125. [Google Scholar] [CrossRef]
- Fu, J.; Kamali-Bernard, S.; Bernard, F.; Cornen, M. Comparison of mechanical properties of C-S-H and portlandite between nano-indentation experiments and a modeling approach using various simulation techniques. Compos. Part B Eng. 2018, 151, 127–138. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Tavakoli, D.; Tarighat, A. Molecular dynamics study on the mechanical properties of Portland cement clinker phases. Comput. Mater. Sci. 2016, 119, 65–73. [Google Scholar] [CrossRef]
- Sanchez, F.; Zhang, L. Interaction energies, structure, and dynamics at functionalized graphitic structure-liquid phase interfaces in an aqueous calcium sulfate solution by molecular dynamics simulation. Carbon N. Y. 2010, 48, 1210–1223. [Google Scholar] [CrossRef]
- Sanchez, F.; Zhang, L. Molecular dynamics modeling of the interface between surface functionalized graphitic structures and calcium-silicate-hydrate: Interaction energies, structure, and dynamics. J. Colloid Interface Sci. 2008, 323, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S.; Crozier, P.; Thompson, A. LAMMPS-large-scale atomic/molecular massively parallel simulator. Sandia Natl. Lab. 2007, 18, 43. [Google Scholar]
- NT BUILD 492. Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments; Nordtest: Espoo, Finland, 1999. [Google Scholar]












| Oxide | Cement | Fly Ash | Slag |
|---|---|---|---|
| CaO | 61.75 | 7.40 | 41.5 |
| SiO2 | 20.64 | 43.9 | 32.2 |
| Al2O3 | 4.62 | 34.8 | 14.6 |
| Fe2O3 | 2.82 | 6.13 | 0.96 |
| K2O | 0.48 | 1.09 | 0.57 |
| MgO | 2.06 | 0.65 | 6.37 |
| Na2O | 0.12 | 0.43 | 0.30 |
| SO3 | 1.20 | 2.00 | 2.12 |
| TiO2 | 0.29 | 1.51 | 0.61 |
| Sample | Cement | Slag | Fly Ash | Water | Sand | Fine Aggregate | Coarse Aggregate | PCE | TIA |
|---|---|---|---|---|---|---|---|---|---|
| 0.4 | 269.5 | 147 | 73.5 | 196 | 702.74 | 404.504 | 606.756 | 0.8‰ | 0% |
| 28 L | 7.546 | 4.116 | 2.058 | 5.444 | 19.677 | 11.326 | 16.989 | 54.88 | 0 g |
| 0.4 | 269.5 | 147 | 73.5 | 196 | 702.74 | 404.504 | 606.756 | 0.8‰ | 0.90% |
| 28 L | 7.546 | 4.116 | 2.058 | 4.882 | 19.677 | 11.326 | 16.989 | 54.88 | 686 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Huang, J. Mechanisms and Critical Technologies of Transport Inhibitor Agent (TIA) throughout C-S-H Nano-Channels. Materials 2022, 15, 515. https://doi.org/10.3390/ma15020515
Luo Q, Huang J. Mechanisms and Critical Technologies of Transport Inhibitor Agent (TIA) throughout C-S-H Nano-Channels. Materials. 2022; 15(2):515. https://doi.org/10.3390/ma15020515
Chicago/Turabian StyleLuo, Qi, and Jiale Huang. 2022. "Mechanisms and Critical Technologies of Transport Inhibitor Agent (TIA) throughout C-S-H Nano-Channels" Materials 15, no. 2: 515. https://doi.org/10.3390/ma15020515