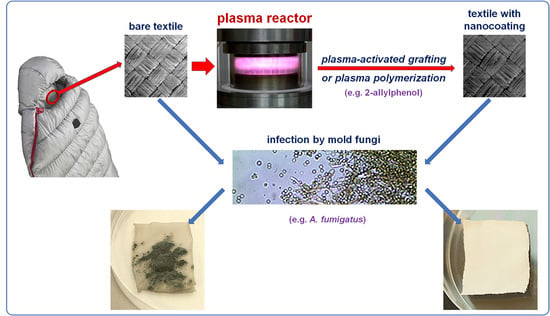
Anti-Mold Protection of Textile Surfaces with Cold Plasma Produced Biocidal Nanocoatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Textile Fabrics
2.2. Mold Fungi
2.3. Antifungal Nanocoating Precursors
2.4. Preparation of Antifungal Nanocoatings
2.5. Characteristics of Nanocoatings
2.6. Microbiological Testing
- 0—No visible growth under the microscope (50×);
- 1—No visible growth to the naked eye, but clearly visible under the microscope;
- 2—Visible growth to the naked eye, coverage ≤ 25% of the area;
- 3—Visible growth to the naked eye, coverage ≤ 50% of the area;
- 4—Extensive growth, coverage > 50% of the area.
3. Results and Discussion
3.1. Antifungal Activity of Precursors
3.2. Structure of Nanocoatings
3.3. Antifungal Activity of Nanocoatings on Textiles
| M-0 | – | Textiles without any antifungal nanocoatings; |
| M-DMAA | – | The nanocoating prepared by plasma-activated grafting of N,N-dimethylallylamine (plasma activation: Ar, 40 W, 120 s; grafting: saturated vapor of 22.8 kPa, 15 min); |
| M-AA+DCP | – | Nanocoating prepared by plasma-activated grafting of allylamine (plasma activation: Ar, 40 W, 120 s; grafting: saturated vapor of 32.3 kPa, 2 h), and then anchoring 3,5-dichlorophenol (Section 2.4); |
| M-AA+TCS | – | Nanocoating prepared by plasma-activated grafting of allylamine (plasma activation: Ar, 40 W, 120 s; grafting: saturated vapor of 32.3 kPa, 2 h), and then anchoring triclosan (Section 2.4); |
| M-APh | – | Nanocoating prepared by plasma polymerization of 2-allylphenol (plasma activation: Ar, 40 W, 120 s; plasma polymerization: mixture of Ar and 2-allylphenol with flow rates of 1.0 and 0.3 sccm, respectively, 8.0 Pa, 5 W, 120 °C, 2 min). |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takai, K.; Ohtsuka, T.; Senda, Y.; Nakao, M.; Yamamoto, K.; Matsuoka, J.; Hirai, Y. Antibacterial properties of antimicrobial-finished textile products. Microbiol. Immunol. 2002, 46, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloid Surf. B-Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Mondal, I.H. (Ed.) Antimicrobial Textiles from Natural Resources; Woodhead Publishing: Duxford, UK, 2021. [Google Scholar]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef] [PubMed]
- Walentowska, J.; Foksowicz-Florczyk, J. Thyme essential oil for antimicrobial protection of natural textiles. Int. Biodeterior. Biodegrad. 2013, 84, 407–411. [Google Scholar] [CrossRef]
- Havrlik, M.; Ryparová, P. Protection of wooden materials against biological attack by using nanotechnology. Acta Polytech. 2015, 55, 101–108. [Google Scholar] [CrossRef]
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Ibrahim, A.; Laquerre, J.; Forcier, P.; Deregnaucourt, V.; Decaens, J.; Vermeersch, O. Antimicrobial agents for textiles: Types, mechanisms and analysis standards. In Textiles for Functional Applications; Kumar, B., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Finley, J.A.; Callow, M.E. The potential of alkyl amines as antifouling biocides, I: Toxicity and structure activity relationships. Biofouling 1996, 9, 257–268. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef]
- Ferreira, L.; Zumbuehl, A. Non-leaching surfaces capable of killing microorganisms on contact. J. Mater. Chem. 2009, 19, 7796–7806. [Google Scholar] [CrossRef]
- Ye, Y.; Song, Q.; Mao, Y. Single-step fabrication of non-leaching antibacterial surfaces using vapor crosslinking. J. Mater. Chem. 2011, 21, 257–262. [Google Scholar] [CrossRef]
- Zahran, M.K.; Ahmed, H.B.; El-Rafie, M.H. Surface modification of cotton fabrics for antibacterial application bycoating with AgNPs–alginate composite. Carbohydr. Polym. 2014, 108, 145–152. [Google Scholar] [CrossRef]
- Xu, Q.B.; Xie, L.J.; Diao, H.; Li, F.; Zhang, Y.Y.; Fu, F.Y.; Lui, X.D. Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr. Polym. 2017, 177, 187–193. [Google Scholar] [CrossRef]
- Xu, Q.B.; Zheng, W.S.; Duan, P.P.; Chen, J.N.; Zhang, Y.Y.; Fu, F.Y.; Diao, H.Y.; Lui, X.D. One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohydr. Polym. 2019, 204, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in sol-gel technology for the coatings of fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedĕla, O.; Slepička, P.; Švorčik, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Haque, A.N.M.A.; Haji, A. Plasma-assisted antimicrobial finishing of textiles: A review. Engineering 2022, 12, 145–163. [Google Scholar] [CrossRef]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; Technomic Publishing: Lancaster, PA, USA, 1996. [Google Scholar]
- Mráček, A.; Lehocký, M.; Smolka, P.; Grulich, O.; Velebný, V. The allylamine grafting on the plasma pre-treated polyester nonwoven fabric: Preparation, characterization and utilization. Fiber. Polym. 2010, 11, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Bilek, F.; Křižová, T.; Lehocký, M. Preparation of active antibacterial LDPE surface through multistep physicochemical approach: I. Allylamine grafting, attachment of antibacterial agent and antibacterial activity assessment. Colloid Surf. B-Biointerfaces 2011, 88, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Bilek, F.; Sulovská, K.; Lehocký, M.; Sáha, P.; Humpoliček, P.; Mozetič, M.; Junkar, I. Preparation of active antibacterial LDPE surface through multistep physicochemical approach II: Graft type effect on antibacterial properties. Colloid Surf. B-Biointerfaces 2013, 102, 842–848. [Google Scholar] [CrossRef]
- Aumsuwan, N.; Heinnhorst, S.; Urban, M.W. Antibacterial surfaces on expanded polytetrafluoroethylene; penicillin attachment. Biomacromolecules 2007, 8, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Aumsuwan, N.; McConnell, M.S.; Urban, M.W. Tunable antimicrobial polypropylene surfaces: Simultaneous attachment of penicillin (gram +) and gentamicin (gram −). Biomacromolecules 2009, 10, 623–629. [Google Scholar] [CrossRef]
- Haji, A.; Mehrizi, M.K.; Akbarpour, R. Optimization of β-cyclodextrin grafting on wool fibers improved by plasma treatment and assessment of antibacterial activity of berberine finished fabric. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 121–133. [Google Scholar] [CrossRef]
- Malshe, P.; Mazloumpour, M.; El-Shafei, A.; Hauser, P. Functional military textile: Plasma-induced graft polymerization of DADMAC for antimicrobial treatment on nylon-cotton blend fabric. Plasma Chem. Plasma Process. 2012, 32, 833–843. [Google Scholar] [CrossRef]
- Widodo, M.; El-Shafei, A.; Hauser, P.J. Surface nanostructuring of Kevlar fibers by atmospheric pressure plasma-induced graft polymerization for multifunctional protective clothing. J. Polym. Sci. Pol. Phys. 2012, 50, 1165–1172. [Google Scholar] [CrossRef]
- Malzahn, K.; Duque, L.; Ciernak, P.; Wiesenmüller, S.; Bender, K.; Förch, R. Antimicrobial activity and cyto-compatibility of plasma polymerized zinc acetylacetonate thin films. Plasma Process. Polym. 2013, 10, 243–249. [Google Scholar] [CrossRef]
- Hegemann, D.; Hossain, M.M.; Balazs, D.J. Nanostructured plasma coatings to obtain multifunctional textile surfaces. Prog. Org. Coat. 2007, 58, 237–240. [Google Scholar] [CrossRef]
- Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliaukas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial properties of diamond-like carbon/silver nanocomposite thin films deposited on textiles: Towards smart bandages. Materials 2016, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Brunon, C.; Chadeau, E.; Oulahal, N.; Grossiord, C.; Dubost, L.; Simon, F.; Bessueille, F.; Degraeve, P.; Leonard, D. Antimicrobial finishing of textiles intended for food processing industry by plasma enhanced chemical vapor deposition—Physical vapor deposition of Ag-SiOCH composites coated with AlxOy or SiOCH encapsulation layers. Thin Solid Films 2017, 628, 132–141. [Google Scholar] [CrossRef]
- Twardowski, A.; Makowski, P.; Małachowski, A.; Hrynyk, R.; Pietrowski, P.; Tyczkowski, J. Plasma Treatment of Thermoactive Membrane Textiles for Superhydrophobicity. Mater. Sci.-Medzg. 2012, 18, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Tyczkowski, J.; Makowski, P.; Twardowski, A.; Małachowski, A.; Pietrowski, P.; Hrynyk, R. Method for Producing Superhydrophobic Nanostructures on the Surface of Textile Materials, with the Use of Plasma. Patent PL 220651, 30 November 2015. [Google Scholar]
- Kapica, R.; Markiewicz, J.; Tyczkowska-Sieroń, E.; Fronczak, M.; Balcerzak, J.; Sielski, J.; Tyczkowski, J. Artificial superhydrophobic and antifungal surface on goose down by cold plasma treatment. Coatings 2020, 10, 904. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kapica, R.; Markiewicz, J.; Małachowski, A.; Małachowski, B. Method for Producing Durable Water-Repellent Layer on the Surface of Natural Down. Patent PL 228924, 19 December 2017. [Google Scholar]
- McDonnell, G.; Russell, D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Tyczkowski, J.; Kierzkowska-Pawlak, H.; Kapica, R.; Balcerzak, J.; Sielski, J. Cold plasma—A promising tool for the production of thin-film nanocatalysts. Catal. Today 2019, 337, 44–54. [Google Scholar] [CrossRef]
- PN EN 14119:2005; Testing of Textiles—Evaluation of the Action of Microfungi. Polish Committee for Standardization Publishing: Warsaw, Poland, 2013.
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. IJoST 2019, 4, 97–118. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.S.; Wang, L.Y.; Wang, Y.N. The investigation of argon plasma surface modification to polyethylene: Quantitative ATR-FTIR spectroscopic analysis. Eur. Polym. J. 2006, 42, 1625–1633. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kierzkowska-Pawlak, H.; Sielski, J.; Krawczyk-Kłys, I. Low-temperature plasma modification of styrene–butadiene block copolymer surfaces for improved adhesion—A kinetic approach. Polymers 2020, 12, 935. [Google Scholar] [CrossRef]
- John Wiley & Sons, Inc. SpectraBase. 2-Allylphenol. Available online: https://spectrabase.com/spectrum/DB1DQpcgs1w (accessed on 25 July 2022).
- Tyczkowski, J.; Kapica, R.; Markiewicz, J.; Tyczkowska-Sieroń, E.; Kiryszewska, A.; Małachowski, A.; Małachowski, B. Method for Producing Antifungal Nanolayer on the Surface of Textile Materials, with the Use of Plasma. Patent PL 232780, 22 March 2019. [Google Scholar]









| Textile | Type of Material |
|---|---|
| T1 | Micro Rip-Stop 100% polyester (both sides) |
| T2 | Micro Rip-Stop inner side—100% polyester outer side—100% polyurethane |
| T3 | 100% natural cotton (both sides) grammage 165 g/m2 |
| T4 | Micro Rip-Stop inner side—100% nylon outer side—100% silicon |
| Precursor | Formula | Characteristic |
|---|---|---|
| Phenols | ||
| 2-Allylphenol (APh) |  | liquid assay 98% b.p. 220 °C |
| 2-Methoxy-4-(2-propenyl)phenol (Eugenol) |  | liquid assay 99% b.p. 254 °C |
| Amines | ||
| Allylamine (AA) |  | liquid assay 98% b.p. 53 °C |
| N,N-Dimethylallylamine (DMAA) |  | liquid assay ≥ 98% b.p. 64 °C |
| 3-Dimethylamino-1-propyne |  | liquid assay 97% b.p. 81 °C |
| Diallyl-dimethylamonium chloride |  | 65 wt.% in H2O |
| Anchored Compounds | ||
| 4-Chloro-2-isopropyl-5- methylphenol |  | crystals assay 99% ethanol: soluble 10% |
| 3,5-Dichlorophenol (DCP) |  | crystals assay 97% ethanol: very soluble |
| 2-Isopropyl-5-methylphenol (Thymol) |  | crystals assay ≥ 98.5% ethanol: soluble 5% |
| 5-Chloro-2-(2,4-dichlorophenoxy) phenol (Triclosan) (TCS) |  | crystals assay 99% ethanol: very soluble |
| Diameter * of The Growth Inhibition Zones [mm] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mold Fungi Species → | A. niger | A. fumigatus | A. tenuissima | P. chrysogenum | ||||
| Precursor concentration [mol/L]→ | 0.01 | 0.1 | 0.01 | 0.1 | 0.01 | 0.1 | 0.01 | 0.1 |
| Incubation time [h]→ | 24 | 24 | 24 | 24 | 72 | 72 | 72 | 72 |
| Precursor type ↓ | ||||||||
| 2-Allylphenol (APh) | 18 | 50 | 10 | 41 | 10 | 56 | 0 | 40 |
| 2-Methoxy-4-(2-propenyl)phenol (Eugenol) | 14 | 43 | 13 | 33 | 10 | 60 | 10 | 37 |
| Allylamine (AA) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N,N-Dimethylallylamine (DMAA) | 8 | 35 | 7 | 27 | 17 | 47 | 0 | 34 |
| 3-Dimethylamino-1-propyne | 8 | 16 | 0 | 7 | 10 | 14 | 0 | 10 |
| Diallyl-dimethylamonium chloride | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4-Chloro-2-isopropyl-5-methyl-phenol | 12 | 28 | 13 | 32 | 18 | 49 | 7 | 15 |
| 3,5-Dichlorophenol (DCP) | 26 | 52 | 18 | 58 | 49 | 82 | 33 | 72 |
| 2-Isopropyl-5-methyl- phenol (Thymol) | 16 | 62 | 14 | 49 | 14 | 49 | 0 | 22 |
| 5-Chloro-2-(2,4-dichlorophenoxy) phenol (Triclosan) (TCS) | 20 | 22 | 22 | 23 | 42 | 44 | 38 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyczkowska-Sieroń, E.; Kiryszewska-Jesionek, A.; Kapica, R.; Tyczkowski, J. Anti-Mold Protection of Textile Surfaces with Cold Plasma Produced Biocidal Nanocoatings. Materials 2022, 15, 6834. https://doi.org/10.3390/ma15196834
Tyczkowska-Sieroń E, Kiryszewska-Jesionek A, Kapica R, Tyczkowski J. Anti-Mold Protection of Textile Surfaces with Cold Plasma Produced Biocidal Nanocoatings. Materials. 2022; 15(19):6834. https://doi.org/10.3390/ma15196834
Chicago/Turabian StyleTyczkowska-Sieroń, Ewa, Agnieszka Kiryszewska-Jesionek, Ryszard Kapica, and Jacek Tyczkowski. 2022. "Anti-Mold Protection of Textile Surfaces with Cold Plasma Produced Biocidal Nanocoatings" Materials 15, no. 19: 6834. https://doi.org/10.3390/ma15196834