Flame Retardancy and Thermal Property of Environment-Friendly Poly(lactic acid) Composites Based on Banana Peel Powder
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
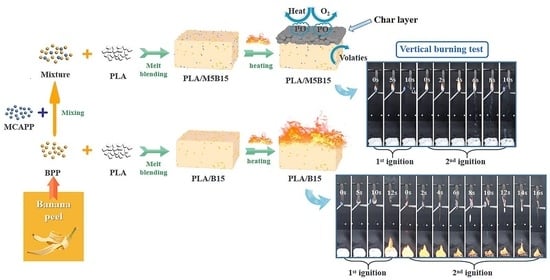
3.1. Flame Retardancy of PLA Composites
3.2. Thermal Properties of PLA Composites
3.3. Morphology of the Char Residues
3.4. Analysis of Pyrolysis Gaseous Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ran, G.W.; Liu, X.D.; Guo, J.; Sun, J.; Li, H.F.; Gu, X.Y.; Zhang, S. Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos. Part B-Eng. 2019, 173, 106772. [Google Scholar] [CrossRef]
- Deghiche, A.; Haddaoui, N.; Zerriouh, A.; Fenni, S.E.; Cavallo, D.; Erto, A.; Benguerba, Y. Effect of the stearic acid-modified TiO2 on PLA nanocomposites: Morphological and thermal properties at the microscopic scale. J. Environ. Chem. Eng. 2021, 9, 106541. [Google Scholar] [CrossRef]
- Guo, J.P.; Tsou, C.H.; De Guzman, M.R.; Wu, C.S.; Zhang, X.M.; Chen, Z.J.; Wen, Y.H.; Yang, T.; Zhuang, Y.J.; Ge, F.F.; et al. Preparation and characterization of bio-based green renewable composites from poly(lactic acid) reinforced with corn stover. J. Polym. Res. 2021, 28, 199. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Z.Q.; Ge, H.D.; Ni, L.K.; Zhang, T.; Huo, S.Q.; Song, P.A.; Fang, Z.P. Core-shell bioderived flame retardants based on chitosan/alginate coated ammonia polyphosphate for enhancing flame retardancy of polylactic acid. ACS Sustain. Chem. Eng. 2020, 8, 6402–6412. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F. Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties. J. Therm. Anal. Calorim. 2021, 146, 131–141. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.X.; Guo, W.W.; Song, L.; Hu, Y. Renewable isosorbide-derived poly(phosphoester) for simultaneously enhanced flame-retardancy and mechanical property of polylactide. Prog. Nat. Sci. 2021, 31, 546–556. [Google Scholar] [CrossRef]
- Jozic, S.P.; Jozic, D.; Jakic, J.; Andricic, B. Preparation and characterization of PLA composites with modified magnesium hydroxide obtained from seawater. J. Therm. Anal. Calorim. 2020, 142, 1877–1889. [Google Scholar] [CrossRef]
- Song, Y.; Zong, X.; Wang, N.; Yan, N.; Shan, X.Y.; Li, J.C. Preparation of gamma-divinyl-3-aminopropyltriethoxysilane modified lignin and its application in flame retardant poly(lactic acid). Materials 2018, 11, 1505. [Google Scholar] [CrossRef]
- Maqsood, M.; Langensiepen, F.; Seide, G. The efficiency of biobased carbonization agent and intumescent flame retardant on flame retardancy of biopolymer composites and investigation of their melt-spinnability. Molecules 2019, 24, 1513. [Google Scholar] [CrossRef]
- Yang, Y.X.; Haurie, L.; Wen, J.H.; Zhang, S.D.; Ollivier, A.; Wang, D.Y. Effect of oxidized wood flour as functional filler on the mechanical, thermal and flame-retardant properties of polylactide biocomposites. Ind. Crops Prod. 2019, 130, 301–309. [Google Scholar] [CrossRef]
- Woo, Y.; Cho, D.W. Effects of ammonium polyphosphate on the flame retarding, tensile, dynamic mechanical, and thermal properties of kenaf fiber/poly(lactic acid) biocomposites fabricated by compression molding. Fibers Polym. 2021, 22, 1388–1396. [Google Scholar] [CrossRef]
- Shukor, F.; Hassan, A.; Islam, M.S.; Mokhtar, M.; Hasan, M. Effect of ammonium polyphosphate on flame retardancy, thermal stability and mechanical properties of alkali treated kenaf fiber filled PLA biocomposites. Mater. Des. 2014, 54, 425–429. [Google Scholar] [CrossRef]
- Bashir, M.; Qayoum, A.; Saleem, S.S. Influence of lignocellulosic banana fiber on the thermal stability of brake pad material. Mater. Res. Express 2019, 6, 115551. [Google Scholar] [CrossRef]
- Kumar, D.V.; Mohan, N.; Bongale, A.K. Fabrication and tribological investigation of Coconut coir/Banana fiber/Glass fiber reinforced hybrid polymer matrix composites—A Taguchi’s approach. Mater. Res. Express 2019, 6, 105345. [Google Scholar] [CrossRef]
- Singh, T.; Lendvai, L.; Dogossy, G.; Fekete, G. Physical, mechanical, and thermal properties of Dalbergia sissoo wood waste-filled poly(lactic acid) composites. Polym. Compos. 2021, 42, 4380–4389. [Google Scholar] [CrossRef]
- Jamadi, A.H.; Razali, N.; Petru, M.; Taha, M.M.; Muhammad, N.; Ilyas, R.A. Effect of chemically treated kenaf fibre on mechanical and thermal properties of PLA composites prepared through fused deposition modeling (FDM). Polymers 2021, 13, 3299. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yu, T.; Chen, S.K.; Li, Y. Soil degradation behavior of ramie/thermoset poly(lactic acid) composites. J. Polym. Res. 2021, 28, 379. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.B.A.; Dai, C.P. Development of biodegradable flame-retardant bamboo charcoal composites, part II: Thermal degradation, gas phase, and elemental analyses. Polymers 2020, 12, 2238. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.A.; Song, L.; Xuan, S.Y.; Xing, W.Y.; Bai, Z.M.; Lu, H.D. Flame retardancy and thermal degradation of intumescent flame retardant poly(lactic acid)/starch biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L. Characterization of Ugandan biomass wastes as the potential candidates towards bioenergy production. Renew. Sustain. Energy Rev. 2020, 117, 109477. [Google Scholar] [CrossRef]
- All About Bananas. Available online: https://www.bananalink.org.uk/all-about-bananas/ (accessed on 18 July 2021).
- Rambo, M.K.D.; Schmidt, F.L.; Ferreira, M.M.C. Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 2015, 144, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.H.; Zhao, Z.L.; Ren, J.M.; Rasool, T.; Naqvi, S.R. Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenergy 2019, 122, 193–201. [Google Scholar] [CrossRef]
- Liao, F.; Yang, L.; Li, Q.; Li, Y.R.; Yang, L.T.; Anas, M.; Huang, D.L. Characteristics and inorganic N holding ability of biochar derived from the pyrolysis of agricultural and forestal residues in the southern China. J. Anal. Appl. Pyrolysis 2018, 134, 544–551. [Google Scholar] [CrossRef]
- Sajna, V.P.; Mohanty, S.; Nayak, S.K. A study on thermal degradation kinetics and flammability properties of poly(lactic acid)/banana fiber/nanoclay hybrid bionanocomposites. Polym. Compos. 2017, 38, 2067–2079. [Google Scholar] [CrossRef]
- Kong, F.B.; He, Q.L.; Peng, W.; Nie, S.B.; Dong, X.; Yang, J.N. Eco-friendly flame retardant poly(lactic acid) composites based on banana peel powders and phytic acid: Flame retardancy and thermal property. J. Polym. Res. 2020, 27, 204. [Google Scholar] [CrossRef]
- Decsov, K.; Bocz, K.; Szolnoki, B.; Bourbigot, S.; Fontaine, G.; Vadas, D.; Marosi, G. Development of bioepoxy resin microencapsulated ammonium-polyphosphate for flame retardancy of polylactic acid. Molecules 2019, 24, 4123. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, W.J.; Gu, X.Y.; Li, H.F.; Liu, X.D.; Sun, J.; Zhang, S. Is there any way to simultaneously enhance both the flame retardancy and toughness of polylactic acid? Polym. Compos. 2019, 40, 932–941. [Google Scholar] [CrossRef]
- Li, S.M.; Ren, J.; Yuan, H.; Yu, T.; Yuan, W.Z. Influence of ammonium polyphosphate on the flame retardancy and mechanical properties of ramie fiber-reinforced poly(lactic acid) biocomposites. Polym. Int. 2010, 59, 242–248. [Google Scholar]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Improving flame retardancy of in-situ silica-epoxy nanocomposites cured with aliphatic hardener: Combined effect of DOPO-based flame-retardant and melamine. Compos. Part C Open Access 2020, 2, 100022. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric analysis properties of cellulosic natural fiber polymer composites: A review on influence of chemical treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Q.; Wang, F.; Zhou, S.J.; Lu, Z.S.; Ran, S.Y.; Li, L.; Shao, J.X. Thermal and mechanical performance of unidirectional composites from bamboo fibers with varying volume fractions. Polym. Compos. 2019, 40, 3929–3937. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Ni, J.X.; Chen, L.J.; Zhao, K.M.; Hu, Y.; Song, L. Preparation of gel-silica/ammonium polyphosphate core-shell flame retardant and properties of polyurethane composites. Polym. Adv. Technol. 2011, 22, 1824–1831. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Dufresne, A.; El-Zawawy, W.K.; Agblevor, F.A. Banana fibers and microfibrils as lignocellulosic reinforcements in polymer composites. Carbohydr. Polym. 2010, 81, 811–819. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Surface treated banana fiber reinforced poly (lactic acid) nanocomposites for disposable applications. J. Clean. Prod. 2013, 52, 392–401. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Hossain, N.; Qureshi, S.S.; Al-Mohaimeed, A.M.; Tanjung, F.A.; Elshikh, M.S.; Siddiqui, M.T.H.; Baloch, H.A.; Mubarak, N.M.; Griffin, G.; et al. Experimental investigation of physicochemical, thermal, mechanical and rheological properties of polylactide/rice straw hydrochar composite. J. Environ. Chem. Eng. 2021, 9, 106011. [Google Scholar] [CrossRef]
- Asaithambi, B.; Ganesan, G.S.; Kumar, S.A. Banana/sisal fibers reinforced poly(lactic acid) hybrid biocomposites; Influence of chemical modification of BSF towards thermal properties. Polym. Compos. 2017, 38, 1053–1062. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Nazir, R.; Lehner, S.; Stampfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Fire and mechanical properties of DGEBA-based epoxy resin cured with a cycloaliphatic hardener: Combined action of silica, melamine and DOPO-derivative. Mater. Des. 2020, 193, 108862. [Google Scholar] [CrossRef]
- Malucelli, G. Surface-engineered fire protective coatings for fabrics through sol-gel and layer-by-layer methods: An overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Jiang, D.; Pan, M.Z.; Cai, X.; Zhao, Y.T. Flame retardancy of rice straw-polyethylene composites affected by in situ polymerization of ammonium polyphosphate/silica. Compos. Part A Appl. Sci. Manuf. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Gao, S.L.; Li, B.; Bai, P.; Zhang, S.Q. Effect of polysiloxane and silane-modified SiO2 on a novel intumescent flame retardant polypropylene system. Polym. Adv. Technol. 2011, 22, 2609–2616. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Wei, R.C.; Yuen, R.K.K.; Chen, W.; Xin, J.H.; Fei, B. Simultaneous fire safety enhancement and mechanical reinforcement of poly(lactic acid) biocomposites with hexaphenyl (nitrilotris(ethane-2,1-diyl))tris (phosphoramidate). J. Hazard. Mater. 2019, 380, 120856. [Google Scholar] [CrossRef]
- Nie, S.B.; Liu, X.L.; Dai, G.L.; Yuan, S.J.; Cai, F.; Li, B.X.; Hu, Y. Investigation on flame retardancy and thermal degradation of flame retardant poly(butylene succinate)/bamboo fiber biocomposites. J. Appl. Polym. Sci. 2012, 125, E485–E489. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.Y.; Jiang, Y.P.; He, H.L.; Liu, H.Y.; Huang, H.; Wan, C.J. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhong, L.; Xu, Y.; Zhang, C.; Wang, P.; Zhang, F.X.; Zhang, G.X. Synthesis of three novel amino acids-based flame retardants with multiple reactive groups for cotton fabrics. Cellulose 2019, 26, 7537–7552. [Google Scholar] [CrossRef]
- Zhou, S.S.; Yang, Y.B.; Zhu, Z.; Xie, Z.H.; Sun, X.Y.; Jia, C.F.; Liu, F.D.; Wang, J.S.; Yang, J.J. Preparation of a halogen-free flame retardant and its effect on the poly (L-lactic acid) as the flame retardant material. Polymer 2021, 229, 124027. [Google Scholar] [CrossRef]









| Samples | Component (wt %) | ||
|---|---|---|---|
| PLA | BPP | MCAPP | |
| PLA | 100 | 0 | 0 |
| PLA/B5 | 95 | 5 | 0 |
| PLA/B10 | 90 | 10 | 0 |
| PLA/B15 | 85 | 15 | 0 |
| PLA/B20 | 80 | 20 | 0 |
| PLA/M5 | 95 | 0 | 5 |
| PLA/M5B15 | 85 | 15 | 5 |
| Determination Basis | Rating | ||
|---|---|---|---|
| V-0 | V-1 | V-2 | |
| Single specimen afterflame time (t1 and t2) 1 | ≤10 s | ≤30 s | ≤30 s |
| Total afterflame time for a set of samples (tf) | ≤50 s | ≤250 s | ≤250 s |
| Afterflame time plus afterglow time of single specimen after second ignition (t2 + t3) | ≤30 s | ≤60 s | ≤60 s |
| Whether the afterflame and/or afterglow spread to the fixture | No | No | No |
| Whether the flame particles or dripping ignite the cotton | No | No | Yes |
| Samples 1 | UL-94 | ||
|---|---|---|---|
| Ignition of Cotton | Dripping 1 | Rating | |
| PLA | Yes | Y/- | No rating |
| PLA/B5 | Yes | Y/Y | V-2 |
| PLA/B10 | Yes | Y/Y | V-2 |
| PLA/B15 | Yes | Y/Y | V-2 |
| PLA/B20 | Yes | Y/Y | V-2 |
| PLA/M5 | No | Y/Y | V-0 |
| PLA/M5B15 | No | N/Y | V-0 |
| Samples | TTI (s) | pHRR (kW·m−2) | tpHRR (s) | av-HRR (kW·m−2) | pCO2y (kg·kg−1) | pCOy (kg·kg−1) |
|---|---|---|---|---|---|---|
| PLA | 54 | 331.4 | 131 | 109.8 | 29.2 | 0.99 |
| PLA/B15 | 29 | 353.8 | 140 | 127.3 | 13.3 | 0.04 |
| PLA/M5B15 | 31 | 296.7 | 113 | 91.4 | 6.3 | 0.02 |
| Samples | T5% (°C) | Tmax (°C) | Rmax (°C) | Residue (wt %) |
|---|---|---|---|---|
| PLA | 341.0 | 373.3 | 4.0 | 1.44 |
| PLA/B15 | 301.7 | 375.0 | 2.9 | 3.26 |
| PLA/B20 | 286.7 | 378.3 | 2.5 | 3.33 |
| PLA/M5B15 (experimental) | 275.0 | 370.4 | 2.7 | 9.32 |
| PLA/M5B15 (theoretical) | 328.5 | 373.3 | 3.6 | 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Nie, B.; Han, C.; Zhao, D.; Hou, Y.; Xu, Y. Flame Retardancy and Thermal Property of Environment-Friendly Poly(lactic acid) Composites Based on Banana Peel Powder. Materials 2022, 15, 5977. https://doi.org/10.3390/ma15175977
Kong F, Nie B, Han C, Zhao D, Hou Y, Xu Y. Flame Retardancy and Thermal Property of Environment-Friendly Poly(lactic acid) Composites Based on Banana Peel Powder. Materials. 2022; 15(17):5977. https://doi.org/10.3390/ma15175977
Chicago/Turabian StyleKong, Fanbei, Baisheng Nie, Chao Han, Dan Zhao, Yanan Hou, and Yuxuan Xu. 2022. "Flame Retardancy and Thermal Property of Environment-Friendly Poly(lactic acid) Composites Based on Banana Peel Powder" Materials 15, no. 17: 5977. https://doi.org/10.3390/ma15175977