Incorporation of Ethylcellulose Microparticles Containing a Model Drug with a Bitter Taste into Nanofibrous Mats by the Electrospinning Technique—Preliminary Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
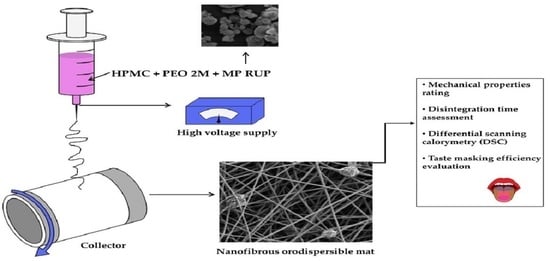
2.2. Formulation of Electrospun Mats with MP Containing RUP
2.3. Preliminary Evaluation of the Obtained Nanofibrous Mats
2.3.1. RUP Content
2.3.2. Mechanical Properties
2.3.3. Disintegration Time Assessment
In Vivo in Healthy Volunteers
In a Petri Dish
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Scanning Electron Microscopy (SEM) Imaging
2.4. Evaluation of Taste-Masking Effectiveness
2.4.1. In Vivo
2.4.2. RUP Dissolution
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery appliccations: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymers 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Scarpaa, M.; Paudelb, A.; Kloproggec, F.; Hsiaob, W.K.; Brescianib, M.; Gaisforda, S.; Orlua, M. Key acceptability attributes of orodispersible films. Eur. J. Pharm. Biopharm. 2018, 125, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, M.; Stegemann, S.; Hsiao, W.K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef]
- Guo, X.; Cun, D.; Wan, F.; Bera, H.; Song, Q.; Tian, X.; Chen, Y.; Rantanen, J.; Yang, M. Comparative assessment of in vitro/in vivo performances of orodispersible electrospun and casting films containing rizatriptan benzoate. Eur. J. Pharm. Biopharm. 2020, 154, 283–289. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wu, J.; Shi, Z.; Zhao, P.; Su, H.; Wang, Q.; Jin, L. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: Nerw delivery system for amlodipine besylate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128096. [Google Scholar] [CrossRef]
- Domokos, A.; Balogh, A.; Dénes, D.; Nyerges, G.; Ződi, L.; Farkas, B.; Marosi, G.; Nagy, Z.K. Continuous manufacturing of orally dissolving webs containing a poorly soluble drug via electrospinning. Eur. J. Pharm. Sci. 2019, 130, 91–99. [Google Scholar] [CrossRef]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the design and development of “fast-dissolving” electrospun nanofibers based drug delivery systems—A systematic review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun orodispersible films of isoniazid for pediatric tuberculosis treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Guo, X.; Sun, Y.; Yang, M. Anti-solvent precipitation method coupled electrospinning process to produce poorly water-soluble drug-loaded orodispersible films. AAPS PharmSciTech 2019, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Rustemkyzy, C.; Belton, P.; Qi, S. Preparation and characterization of ultra rapidly dissolving orodispersible films for treating and preventing iodine deficiency in the pediatric population. J. Agric. Food Chem. 2015, 63, 9831–9838. [Google Scholar] [CrossRef] [PubMed]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009. [Google Scholar]
- FDA Inactive Ingredients Database. Available online: https://search.fda.gov/search?utf8=%E2%9C%93&affiliate=fda1&query=ethylcellulose&commit=Search (accessed on 10 May 2022).
- Safety & Toxicity of Excipients for Paediatrics, STEP Database. Available online: http://www.eupfi.org/step-database-info/ (accessed on 10 May 2022).
- Aydogdu, A.; Sumnu, G.; Sahin, S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohydr. Polym. 2018, 1, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, D.-G.; Zhang, L.-L.; Liu, X.-K.; Deng, Y.-C.; Zhao, M. Electrospun hypromellose-based hydrophilic composites for rapid dissolution of poorly water-soluble drug. Carbohydr. Polym. 2017, 174, 617–625. [Google Scholar] [CrossRef]
- Nazari, K.; Kontogiannidou, E.; Ahmad, R.H.; Gratsani, A.; Rasekh, M.; Arshad, M.S.; Sunar, B.S.; Armitage, D.; Bouropoulos, N.; Chang, M.-W. Development and characterization of cellulose based electrospun mats for buccal delivery of non-steroidal anti-inflammatory drug (NSAID). Eur. J. Pharm. Sci. 2017, 102, 147–155. [Google Scholar] [CrossRef]
- Mullol, J.; Gonzalez-Nunez, V.; Bachert, C. Rupatadine: Global safety evaluation in allergic rhinitis and urticaria. Expert Opin. Drug Saf. 2016, 15, 1439–1448. [Google Scholar]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.D.; Selomuyla, C. On the spray drying of uniform functional microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Wasilewska, K.; Szekalska, M.; Ciosek-Skibinska, P.; Lenik, J.; Basa, A.; Jacyna, J.; Markuszewski, M.; Winnicka, K. Ethylcellulose in organic solution or aqueous dispersion form in designing taste-masked microparticles by the spray drying technique with a model bitter drug: Rupatadine fumarate. Polymers 2019, 11, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudekar, R.L.; Mahajan, M.P.; Sawant, S.D. Validated RP-HPLC method for the estimation of rupatadine fumarate in bulk and tablet dosage form. Pharma Chem. 2012, 4, 1047–1053. [Google Scholar]
- Redasani, V.K.; Kothawade, A.R.; Surana, S.J. Stability indicating RP-HPLC method for simultaneous estimation of rupatadine fumarate and montelukast sodium in bulk and tablet dosage form. J. Anal. Chem. 2014, 69, 384–389. [Google Scholar] [CrossRef]
- Rele, R.V.; Mali, R.N. New validated RP-HPLC method for quantification of rupatadine fumarate impurities in solid dosage form supported by forced degradation studies. Der Pharm. Lett. 2016, 8, 66–72. [Google Scholar]
- Olechno, K.; Maciejewski, B.; Głowacz, K.; Lenik, J.; Ciosek-Skibińska, P.; Basa, A.; Winnicka, K. Orodispersible films with rupatadine fumarate enclosed in ethylcellulose microparticles as drug delivery platform with taste-masking effect. Materials 2022, 15, 2126. [Google Scholar] [CrossRef]
- Filip, P.; Peer, P. Characterization of poly(ethylene oxide) nanofibers—Mutual relations between mean diameter of electrospun nanofibers and solution characteristics. Processes 2019, 12, 948. [Google Scholar] [CrossRef] [Green Version]
- Henríquez, L.C.; Redondo, G.M.; Zúñiga, R.V.; Berrocal, G.C. Identification of rupatadine fumarate polymorphic crystalline forms in pharmaceutical raw materials. Asian J. Sci. Technol. 2018, 9, 7482–7487. [Google Scholar]
- Henríquez, L.C.; Zúñiga, R.V.; Redondo, G.M.; Berrocal, G.C.; Vargas, G.H. Determination of the impact caused by direct compression on the crystalline state of rupatadine fumarate 10 mg tablets. Int. J. Pharm. Technol. Biotechnol. 2019, 6, 1–12. [Google Scholar]
- The European Pharmacopoeia, 10th ed.; Council of Europe: Strasburg, France, 2019.
- The United States Pharmacopeia and National Formulary (USP41-NF 36); Pharmacopeia Convention: Rockville, MD, USA, 2018; Volume 2.
- Preis, M.; Pein, M.; Breitkreutz, J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics 2012, 4, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al–Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. Mechanical Properties of Solid Polymers, 3rd ed.; John Wiley & Sons, Ltd.: Hobokoen, NJ, USA, 2012. [Google Scholar]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible films and tablets with prednisolone microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef] [PubMed]
- ISO Standards DIN EN ISO-527-1; Plastics—General Principles of the Determination of Ten Sile Properties. Din Deutsch Institut für Normung: Berlin, Germany, 1996.
- ISO Standards DIN EN ISO-527-2; Plastics—Determination of Ten Sile Properties. Din Deutsch Institut für Normung: Berlin, Germany, 1996.
- ISO Standards DIN EN ISO-527-3; Plastics—Determination of Tensile Properties. Din Deutsch Institut für Normung: Berlin, Germany, 2002.
- ASTM D882-02; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM D638-14; Standard Test Methods for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2015.
- Visser, J.C.; Dohmen, W.M.C.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef] [PubMed]



| Formulation | Tear Resistance [N] | Tensile Strength [N/mm2] | Elongation at Break [%] | Young’s Modulus [MPa] | Disintegration Time [s] |
|---|---|---|---|---|---|
| F1 | 3.14 ± 0.31 | 3.92 ± 0.015 | 5.1 ±0.03 | 220.0 ±0.72 | <30 s |
| F2 | 1.67 ± 0.34 | 2.08 ± 0.01 | 5.0 ± 0.03 | 179.0 ± 0.58 | <30 s |
| F3 | 1.37 ± 0.27 | 1.71 ± 0.06 | 4.0 ± 0.05 | 169.0 ±0.69 | <30 s |
| Volunteer/ Formulation | Score | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| A | 0 | 3 | 1 |
| B | 0 | 2 | 0 |
| C | 0 | 3 | 1 |
| D | 0 | 3 | 1 |
| E | 0 | 2 | 0 |
| F | 0 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olechno, K.; Grilc, N.K.; Zupančič, Š.; Winnicka, K. Incorporation of Ethylcellulose Microparticles Containing a Model Drug with a Bitter Taste into Nanofibrous Mats by the Electrospinning Technique—Preliminary Studies. Materials 2022, 15, 5286. https://doi.org/10.3390/ma15155286
Olechno K, Grilc NK, Zupančič Š, Winnicka K. Incorporation of Ethylcellulose Microparticles Containing a Model Drug with a Bitter Taste into Nanofibrous Mats by the Electrospinning Technique—Preliminary Studies. Materials. 2022; 15(15):5286. https://doi.org/10.3390/ma15155286
Chicago/Turabian StyleOlechno, Katarzyna, Nina Katarina Grilc, Špela Zupančič, and Katarzyna Winnicka. 2022. "Incorporation of Ethylcellulose Microparticles Containing a Model Drug with a Bitter Taste into Nanofibrous Mats by the Electrospinning Technique—Preliminary Studies" Materials 15, no. 15: 5286. https://doi.org/10.3390/ma15155286