Preparation and Characterization of Novel Microgels Containing Nano-SiO2 and Copolymeric Hydrogel Based on Poly (Acrylamide) and Poly (Acrylic Acid): Morphological, Structural and Swelling Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
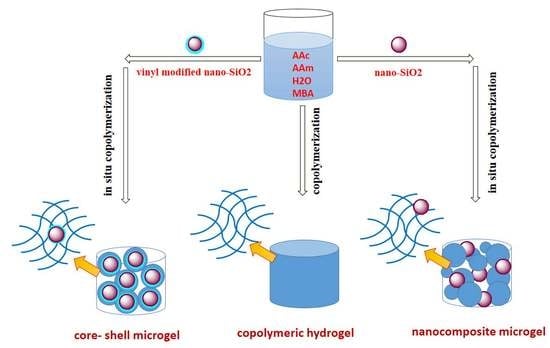
2.2. Synthesis of Nanosilica Functional Monomer
2.3. Synthesis of Core—Shell Microgels
2.4. Synthesis of Nanocomposite Microgels
2.5. Prepration of Polyacrylamide and Copolymeric Hydrogel
2.6. Dynamic Swelling Studies
2.7. Methods
3. Results and Discussion
3.1. Morphological Studies
3.2. FTIR Analysis
3.3. Thermal Stability Analysis
3.4. Swelling Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erceg, T.; Dapčević-Hadnađev, T.; Hadnađev, M.; Ristić, I. Swelling kinetics and rheological behaviour of microwave synthesized poly(acrylamide-co-acrylic acid) hydrogels. Colloid Polym. Sci. 2020, 299, 11–23. [Google Scholar] [CrossRef]
- Sorkhabi, T.S.; Samberan, M.F.; Ostrowski, K.A.; Majka, T.M. Novel Synthesis, Characterization and Amoxicillin Release Study of pH-Sensitive Nanosilica/Poly(acrylic acid) Macroporous Hydrogel with High Swelling. Materials 2022, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties-A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Sun, N.; Ji, R.; Zhang, F.; Song, X.; Xie, A.; Liu, J.; Zhang, M.; Niu, L.; Zhang, S. Structural evolution in poly(acrylic-co-acrylamide) pH-responsive hydrogels by low-field NMR. Mater. Today Commun. 2019, 22, 100748. [Google Scholar] [CrossRef]
- De Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.-U. Insight into hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Atchudan, R.; Edison, T.; Babu, R.; Karpagavinayagam, P.; Vedhi, C. A Short Review on Recent Advances of Hydrogel-Based Adsorbents for Heavy Metal Ions. Metals 2021, 11, 864. [Google Scholar] [CrossRef]
- Haque, M.O.; Mondal, M.I.H. Cellulose-Based Hydrogel for Personal Hygiene Applications. In Cellulose-Based Superabsorbent Hydrogels; Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Tomić, S.; Nikodinović-Runić, J.; Vukomanović, M.; Babić, M.M.; Vuković, J.S. Novel Hydrogel Scaffolds Based on Alginate, Gelatin, 2-Hydroxyethyl Methacrylate, and Hydroxyapatite. Polymers 2021, 13, 932. [Google Scholar] [CrossRef]
- Tran, N.-P.-D.; Yang, M.-C. Synthesis and Characterization of Silicone Contact Lenses Based on TRIS-DMA-NVP-HEMA Hydrogels. Polymers 2019, 11, 944. [Google Scholar] [CrossRef] [Green Version]
- Ehrenhofer, A.; Binder, S.; Gerlach, G.; Wallmersperger, T. Multisensitive Swelling of Hydrogels for Sensor and Actuator Design. Adv. Eng. Mater. 2020, 22, 2000004. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Q.; Li, H.; Ma, M.; Zhang, N. Stretchable and Shelf-Stable All-Polymer Supercapacitors Based on Sealed Conductive Hydrogels. ACS Appl. Energy Mater. 2020, 3. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Liu, Z.; Li, W.; Liu, Y.; Li, J.; Wei, H.; Han, H. Fabrication of a water-retaining, slow-release fertilizer based on nanocomposite double-network hydrogels via ion-crosslinking and free radical polymerization. J. Ind. Eng. Chem. 2020, 93, 375–382. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, Y.; Yang, G.; Zhang, A. Water- and Fertilizer-Integrated Hydrogel Derived from the Polymerization of Acrylic Acid and Urea as a Slow-Release N Fertilizer and Water Retention in Agriculture. J. Agric. Food Chem. 2018, 66, 5762–5769. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A.K. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef]
- Krafcik, M.J.; Macke, N.D.; Erk, K.A. Improved Concrete Materials with Hydrogel-Based Internal Curing Agents. Gels 2017, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zhang, B.; Cong, Q.; He, X.; Gao, M.; Li, G. Organic/inorganic nanocomposites of ZnO/CuO/chitosan with improved properties. Mater. Chem. Phys. 2016, 178, 88–97. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Meshram, I.; Kanade, V.; Nandanwar, N.; Ingle, P. Super-Absorbent Polymer: A Review on the Characteristics and Application. Int. J. Adv. Res. Chem. Sci. 2020, 7, 8–21. [Google Scholar] [CrossRef]
- Lv, Q.; Shen, Y.; Qiu, Y.; Wu, M.; Wang, L. Poly(acrylic acid)/poly(acrylamide) hydrogel adsorbent for removing methylene blue. J. Appl. Polym. Sci. 2020, 137, 49322. [Google Scholar] [CrossRef]
- Sen, M. Nanocomposite Materials. Nanotechnology and the Environment; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Guo, Z.-X. The influence of interphase on nylon-6/nano-SiO2 composite materials obtained fromin situ polymerization. Polym. Int. 2003, 52, 981–986. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Q. Preparation of conductive polyaniline/nanosilica particle composites through ultrasonic irradiation. J. Appl. Polym. Sci. 2003, 87, 1811–1817. [Google Scholar] [CrossRef]
- Rueda, L.I.; Anton, C.C. Effect of the textural characteristics of the new silicas on the dynamic properties of styrene-butadiene rubber (SBR) vulcanizates. Polym. Compos. 1988, 9, 204–208. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Wang, M.; Shen, Z. The effects of atomic oxygen on polyimide resin matrix composite containing nano-silicon dioxide. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 243, 320–324. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, P.; Tian, X.; Zheng, K. Pyrolysis studies of polyethylene terephthalate/silica nanocomposites. J. Appl. Polym. Sci. 2006, 104, 9–14. [Google Scholar] [CrossRef]
- Ali, A.E.-H.; Shawky, H.; El Rehim, H.A.; Hegazy, E. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003, 39, 2337–2344. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.D.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2 nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorkhabi, T.S.; Samberan, M.F.; Ostrowski, K.A.; Majka, T.M.; Piechaczek, M.; Zajdel, P. Preparation and Characterization of Novel Microgels Containing Nano-SiO2 and Copolymeric Hydrogel Based on Poly (Acrylamide) and Poly (Acrylic Acid): Morphological, Structural and Swelling Studies. Materials 2022, 15, 4782. https://doi.org/10.3390/ma15144782
Sorkhabi TS, Samberan MF, Ostrowski KA, Majka TM, Piechaczek M, Zajdel P. Preparation and Characterization of Novel Microgels Containing Nano-SiO2 and Copolymeric Hydrogel Based on Poly (Acrylamide) and Poly (Acrylic Acid): Morphological, Structural and Swelling Studies. Materials. 2022; 15(14):4782. https://doi.org/10.3390/ma15144782
Chicago/Turabian StyleSorkhabi, Tannaz Soltanolzakerin, Mehrab Fallahi Samberan, Krzysztof Adam Ostrowski, Tomasz M. Majka, Marcin Piechaczek, and Paulina Zajdel. 2022. "Preparation and Characterization of Novel Microgels Containing Nano-SiO2 and Copolymeric Hydrogel Based on Poly (Acrylamide) and Poly (Acrylic Acid): Morphological, Structural and Swelling Studies" Materials 15, no. 14: 4782. https://doi.org/10.3390/ma15144782