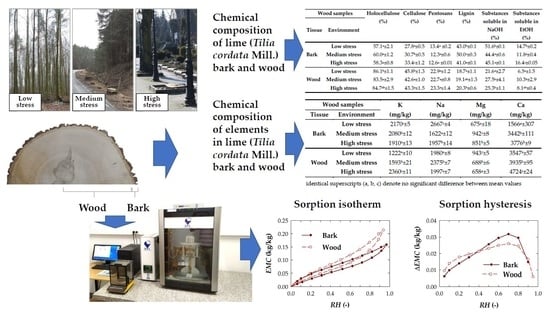
Chemical Composition and Related Properties of Lime (Tilia cordata Mill.) Bark and Wood as Affected by Tree Growth Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical Analysis
2.3. Chemical Composition of Bark and Wood
2.4. Determination of Chemical Elements
2.5. Sorption Experiments
2.6. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.1.1. Chemical Composition of Bark and Wood
3.1.2. Determination of the Chemical Elements of Bark and Wood
3.2. Sorption Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samojlik, T. Drzewo wielce użyteczne–historia lipy drobnolistnej (Tilia cordata) w Puszczy Białowieskiej. Rocz. Dendrol. 2005, 53, 55–64. [Google Scholar]
- Hedemann, O. Dzieje Puszczy Białowieskiej w Polsce przedrozbiorowej (w Okresie do 1798 Roku); Instytut Badawczy Lasów Państwowych: Warszawa, Poland, 1939. [Google Scholar]
- Szretter, P. Rys historyczny powstania w Puszczy Białowieskiej w roku 1831; Wyd. Chocieszyński: Poznań, Poland, 1893; p. 81. [Google Scholar]
- Połujański, A. Opisanie lasów Królestwa Polskiego i guberni zachodnich cesarstwa rosyjskiego pod względem historycznym, statystycznym i gospodarczym; J. Unger: Warszawa, Poland, 1854; Volume 2. [Google Scholar]
- Martynova, M.; Sultanova, R.; Odintsov, G.; Sazgutdinova, R.; Khanova, E. Growth of Tilia cordata Mill. in Urban Forests. South-East Eur. For. 2020, 11, 51–59. [Google Scholar] [CrossRef]
- Sadowiec, K.J.; Gawroński, S.W. Przydatność wybranych gatunków lip (Tilia sp.) do fitoremediacji powietrza z zanieczyszczeń pyłowych. Woda Sr. Obsz. Wiej. 2013, 13, 131–148. [Google Scholar]
- Pigott, C.D. Tilia Cordata Miller. J. Ecol. 1991, 79, 1147–1207. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; de Rigo, D. Tilia cordata, Tilia platyphyllos and other limes in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Office of the European Union: Luxembourg, 2016; p. e010ec5+. [Google Scholar]
- Pásztory, Z.; Mohácsiné, I., R.; Gorbacheva, G.; Börcsök, Z. Utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Giannotas, G.; Kamperidou, V.; Barboutis, I. Tree bark utilization in insulating bio-aggregates: A review. Biofuels Bioprod. Bioref. 2021, 15, 1989–1999. [Google Scholar] [CrossRef]
- Kosiorek, M.; Modrzewska, B.; Wyszkowski, M. Levels of selected trace elements in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula L.), and Norway maple (Acer platanoides L.) in an urbanized environment. Environ. Monit. Assess 2016, 188, 598. [Google Scholar] [CrossRef] [Green Version]
- Krutul, D.; Zielenkiewicz, T.; Zawadzki, J.; Radomski, A.; Antczak, A.; Drożdżek, M. Influence of urban environment originated heavy metal pollution on the extractives and mineral substances content in bark and wood of oak (Quercus robur L.). Wood Res. 2014, 59, 177–190. [Google Scholar]
- Pavlović, D.; Pavlović, M.; Marković, M.; Karadžić, B.; Kostić, O.; Jarić, S. Possibilities of assessing trace metal pollution using Betula pendula Roth. leaf and bark-experience in Serbia. J. Serbian Chem. Soc. 2017, 82, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Krutul, D.; Antczak, A.; Radomski, A.; Wójcik, R.; Drożdżek, M.; Zawadzki, J. Porównanie składu chemicznego kory szybko rosnącej topoli z korą innych gatunków drzew liściastych. Sylwan 2020, 164, 767–774. [Google Scholar]
- Pańczyk, M.; Świsłowski, P.; Rajfur, M. Ocena jednorodności zanieczyszczenia kory drzew liściastych metalami ciężkimi. Proc. ECOpole 2018, 12, 54. [Google Scholar]
- Grochowski, W. Uboczna Produkcja Leśna; PWN: Warszawa, Poland, 1976; p. 571. [Google Scholar]
- Krzysik, F. Nauka o Drewnie, 4th ed.; PWN: Warszawa, Poland, 1975; p. 656. [Google Scholar]
- Chrabąszcz, M.; Mróz, L. Tree Bark, a valuable source of information on air quality. Pol. J. Environ. Stud. 2017, 26, 453–466. [Google Scholar] [CrossRef]
- Orlovskaya, T.V.; Gyulbyakova, K.N.; Guzhva, N.N.; Ogurtsov, Y.A. Studying the Tilia cordata L. bark with the purpose of creation the new medicines. Mod. Probl. Sci. Educ. 2013, 2, 4–27. [Google Scholar]
- Jabeur, I.; Martins, N.; Barros, L.; Calhelha, R.C.; Vaz, J.; Achour, L.; Santos-Buelga, C.; Ferreira, I.C. Contribution of the phenolic composition to the antioxidant, anti-inflammatory and antitumor potential of Equisetum giganteum L. and Tilia platyphyllos Scop. Food Funct. 2017, 8, 975–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, H.M.E.; Rasmussen, H.N. Bark extract influence on spore germination in corticolous lichen Xanthoria parietina in vitro. Mycol. Prog. 2021, 20, 313–323. [Google Scholar] [CrossRef]
- Majka, J.; Olek, W. Sorption properties of mature and juvenile lime wood (Tilia sp.). Folia For. Pol. B 2008, 39, 65–75. [Google Scholar]
- Hailwood, A.; Horrobin, S. Absorption of water by polymers: Analysis in terms of a simple model. Trans. Faraday Soc. 1946, 42, B084–B092. [Google Scholar] [CrossRef]
- Simpson, W.T. Sorption theories applied to wood. Wood Fiber 1980, 12, 183–195. [Google Scholar]
- Kusiak, W.; Majka, J.; Ratajczak, I.; Górska, M.; Zborowska, M. Evaluation of Environmental Impact on Selected Properties of Lime (Tilia Cordata Mill.) Wood. Forests 2020, 11, 746. [Google Scholar] [CrossRef]
- Rémond, R.; Barros, L.C.A.; Almeida, G. Moisture Transport and Sorption in Beech and Spruce Barks. Holzforschung 2018, 72, 105–111. [Google Scholar] [CrossRef]
- Rahimi, S.; Singh, K.; DeVallance, D.; Chu, D.; Bahmani, M. Drying Behavior of Hardwood Components (Sapwood, Heartwood, and Bark) of Red Oak and Yellow-Poplar. Forests 2022, 13, 722. [Google Scholar] [CrossRef]
- Browning, B.L. The Chemistry of Wood; Interscience Publishers: New York, NY, USA, 1967. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. Pentosans in Wood and Pulp, T 223 cm-01; Technical Association of the Pulp and Paper Industry: New York, NY, USA, 2001; p. 5. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. Acid Insoluble Lignin in Wood and Pulp, T 222 cm-06; Technical Association of the Pulp and Paper Industry: New York, NY, USA, 2006; p. 5. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. Solvent Extractives of Wood and Pulp, T 204 cm-97; Technical Association of the Pulp and Paper Industry: New York, NY, USA, 2007; p. 12. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. One Percent Sodium Hydroxide Solubility of Wood and Pulp, T 212 cm-02; Technical Association of the Pulp and Paper Industry: New York, NY, USA, 2002; p. 6. [Google Scholar]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A. The general mechanism of water sorption on foodstuffs–Importance of the multitemperature fitting of data and the hierarchy of models. J. Food Eng. 2007, 82, 528–535. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A.; Rychlicki, G. Applicability of the generalised D’Arcy and Watt model to description of water sorption on pineapple and other foodstuffs. J. Food Eng. 2007, 79, 718–723. [Google Scholar] [CrossRef]
- Majka, J.; Czajkowski, Ł.; Olek, W. Effects of cyclic changes in relative humidity on the sorption hysteresis of thermally modified spruce wood. BioResources 2016, 11, 5265–5275. [Google Scholar] [CrossRef] [Green Version]
- Wagenführ, R.; Scheiber, C. Holzatla; Fachbuchverlag: Leipzig, Germany, 1985; pp. 1–690. [Google Scholar]
- Rowell, R.M.; Pettersen, R.; Tshabalala, M.A. Cell Wall Chemistry from: Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 35–74. [Google Scholar]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A. Some remarks on the classification of water vapor sorption isotherms and Blahovec and Yanniotis isotherm equation. Dry. Technol. 2011, 29, 984–991. [Google Scholar] [CrossRef]
- Brack, C.L. Pollution mitigation and carbon sequestration by an urban forest. Environ. Pollut. 2002, 116, S195–S200. [Google Scholar] [CrossRef]
- Eberhard, T. Impact of Industrial Source on the Chemical Composition of Loblolly Pine Bark. For. Prod. J. 2012, 62, 516–519. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Janz, D.; Zienkiewicz, K.; Herrfurth, C.; Feussner, I.; Chen, S.; Polle, A. Wood Formation under Severe Drought Invokes Adjustment of the Hormonal and Transcriptional Landscape in Poplar. Int. J. Mol. Sci. 2021, 13, 9899. [Google Scholar] [CrossRef] [PubMed]
- Paes de Melo, B.; Carpinetti, P.d.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M.F.d.S. Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M. Antifungal activities of wood extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Functionalized polymers from lignocellulosic biomass: State of the art. Polymers 2013, 5, 600–642. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, Z.; Yameen, M.; Jahangeer, M.; Riaz, M.; Ghaffar, A.; Javid, I. Lignin as Natural Antioxidant Capacity; Poletto, M., Ed.; In Lignin-Trends and Applications; Intech Open: London, UK, 2018. [Google Scholar]
- Kovářová, M.; Pyszko, P.; Plášek, V. How Does the pH of Tree Bark Change with the Presence of the Epiphytic Bryophytes from the Family Orthotrichaceae in the Interaction with Trunk Inclination? Plants 2022, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Österås, A.H.; Greger, M. Accumulation of, and interactions between, calcium and heavy metals in wood and bark of Picea abies. Z. Pflanzenernähr. Bodenk. 2003, 166, 246–253. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P.; Wilk, R.; Szwajgier, D.; Szymanowska, D. Prunus padus L. bark as a functional promoting component in functional herbal infusions—Cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects. Open Chem. 2021, 19, 1052–1061. [Google Scholar] [CrossRef]
- Simón, C.; Esteban, L.G.; de Palacios, P.; Fernández, F.G.; Martín-Sampedro, R.; Eugenio, M.E. Thermodynamic analysis of water vapour sorption behaviour of juvenile and mature wood of Abies alba Mill. J. Mater. Sci. 2015, 50, 7282–7292. [Google Scholar] [CrossRef]
- Jankowska, A.; Drożdżek, M.; Sarnowski, P.; Horodeński, J. Effect of extractives on the equilibrium moisture content and shrinkage of selected tropical wood species. BioResources 2017, 12, 597–607. [Google Scholar] [CrossRef]
- Vahtikari, K.; Rautkari, L.; Noponen, T.; Lillqvist, K.; Hughes, M. The influence of extractives on the sorption characteristics of Scots pine (Pinus sylvestris L.). J. Mater. Sci. 2017, 52, 10840–10852. [Google Scholar] [CrossRef]
- Hernández, R.E. Moisture sorption properties of hardwoods as affected by their extraneous substances, wood density, and interlocked grain. Wood Fiber Sci. J. Soc. Wood Sci. Technol. 2007, 39, 132–145. [Google Scholar]
- Spalt, H.A. Water vapor sorption by wood. For. Prod. J. 1958, 8, 288–295. [Google Scholar]



| Wood Samples | Holocellulose (%) | Cellulose (%) | Pentosans (%) | Lignin (%) | Substances Soluble in NaOH (%) | Substances Soluble in EtOH (%) | |
|---|---|---|---|---|---|---|---|
| Tissue | Environment | ||||||
| Bark | Low stress | 57.1 a ± 2.1 | 27.8 a ± 0.5 | 13.4 a ± 0.2 | 43.0 b ± 0.1 | 51.6 b ± 0.1 | 14.7 b ± 0.2 |
| Medium stress | 60.0 a ± 1.2 | 30.7 b ± 0.5 | 12.3 a ± 0.6 | 50.0 c ± 0.3 | 44.4 a ± 0.6 | 11.8 a ± 0.4 | |
| High stress | 58.3 a ± 0.8 | 33.4 c ± 1.2 | 12.6 a ± 0.01 | 41.0 a ± 0.1 | 45.1 a ± 0.1 | 16.4 c ± 0.05 | |
| Wood | Low stress | 86.1 b ± 1.1 | 45.8 b ± 1.3 | 22.9 a ± 1.2 | 18.7 a ± 1.1 | 21.6 a ± 2.7 | 6.5 a ± 1.5 |
| Medium stress | 83.5 a ± 2.9 | 42.6 a ± 1.0 | 22.7 a ± 0.8 | 19.1 a,b ± 1.3 | 27.5 b ± 4.1 | 10.3 b ± 2.9 | |
| High stress | 84.7 a,b ± 1.5 | 43.3 a ± 1.5 | 23.3 a ± 1.4 | 20.3 b ± 0.6 | 25.3 b ± 1.1 | 8.1 a,b ± 0.4 | |
| Wood Samples | K (mg/kg) | Na (mg/kg) | Mg (mg/kg) | Ca (mg/kg) | |
|---|---|---|---|---|---|
| Tissue | Environment | ||||
| Bark | Low stress | 2170 c ± 5 | 2667 c ± 4 | 675 a ± 18 | 1566 a ± 307 |
| Medium stress | 2080 b ± 12 | 1622 a ± 12 | 942 c ± 8 | 3442 b ± 111 | |
| High stress | 1910 a ± 13 | 1957 b ± 14 | 851 b ± 5 | 3776 b ± 9 | |
| Wood | Low stress | 1222 a ± 10 | 1980 a ± 8 | 943 c ± 5 | 3547 a ± 57 |
| Medium stress | 1593 b ± 21 | 2375 b ± 7 | 688 b ± 6 | 3935 b ± 95 | |
| High stress | 2360 c ± 11 | 1997 a ± 7 | 658 a ± 3 | 4724 c ± 24 | |
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| Wood Samples | Fe (mg/kg) | Zn (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Cd (mg/kg) | ||
| Tissue | Environment | ||||||
| (b) | |||||||
| Wood Samples | B (mg/kg) | Ni (mg/kg) | Cr (mg/kg) | Al (mg/kg) | As (µg/kg) | Hg (µg/kg) | |
| Tissue | Environment | ||||||
| Bark | Low stress | 160.5 a ± 4.4 | 13.6 a ± 0.3 | 5.74 b ± 0.22 | 0.050 a ± 0.01 | 0.016 a ± 0.004 | |
| Medium stress | 336.7 c ± 1.3 | 15.8 b ± 0.1 | 4.59 a ± 0.01 | 0.096 a ± 0.01 | 0.304 b ± 0.011 | ||
| High stress | 242.2 b ± 1.1 | 18.0 c ± 0.3 | 5.28 b ± 0.22 | 1.626 b ± 0.21 | 0.609 c ± 0.075 | ||
| Wood | Low stress | 187.3 c ± 1.7 | 25.3 b ± 0.3 | 9.0 b ± 0.15 | 7.96 a ± 0.27 | 0.138 a ± 0.0120 | |
| Medium stress | 115.0 a ± 0.4 | 34.5 c ± 0.1 | 16.9 c ± 0.14 | 26.5 c ± 0.34 | 0.48 b ± 0.010 | ||
| High stress | 132.5 b ± 0.8 | 24.0 a ± 0.1 | 6.8 a ± 0.17 | 10.1 b ± 0.30 | 0.75 c ± 0.012 | ||
| Low stress | 15.6 c ± 0.4 | 0.45 a ± 0.04 | 2.42 a ± 0.14 | 724 b ± 3 | 0.012 a ± 0.0003 | 0.025 a±0.0007 | |
| Bark | Medium stress | 4.3 b ± 0.5 | 0.02 a ± 0.01 | 3.95 b ± 0.20 | 792 c ± 14 | 0.015 b ± 0.0001 | 0.030 b±0.0001 |
| High stress | 0.5 a ± 0.1 | 3.92 b ± 0.35 | 7.50 c ± 0.29 | 687 a ± 8 | 0.018 c ± 0.0003 | 0.035c±0.0006 | |
| Low stress | 19.1 a ± 0.1 | 5.70 b ± 0.20 | 5.14 a ± 0.04 | 787 c ± 10 | 0.027 b ± 0.0003 | 0.044 a±0.0170 | |
| Wood | Medium stress | 11.6 b ± 0.4 | 0.53 a ± 0.02 | 4.88 a ± 0.19 | 720 b ± 6 | 0.037 c ± 0.0001 | 0.073 b±0.0002 |
| High stress | 1.59 a ± 0.12 | 5.99 c ± 0.03 | 8.79 b ± 0.10 | 611 a ± 3 | 0.024 a ± 0.0001 | 0.041 a±0.0006 | |
| Wood Samples | Sorption Phase | m (kg/kg) | K | k | w | R2 | |
|---|---|---|---|---|---|---|---|
| Tissue | Environment | ||||||
| Bark | Low stress | Ads. | 0.1017 | 3.0272 | 0.8813 | 0.3594 | 0.99998 |
| Des. | 0.0931 | 7.2482 | 0.7851 | 0.5883 | 0.99992 | ||
| Medium stress | Ads. | 0.1153 | 1.6339 | 0.8695 | 0.2637 | 0.99992 | |
| Des. | 0.0740 | 5.5129 | 0.6055 | 1.2209 | 0.99995 | ||
| High stress | Ads. | 0.1292 | 1.3830 | 0.8441 | 0.2566 | 0.99999 | |
| Des. | 0.0655 | 6.9779 | 0.4307 | 2.5057 | 0.99991 | ||
| Wood | Low stress | Ads. | 0.0811 | 2.6942 | 0.8654 | 0.6059 | 0.99995 |
| Des. | 0.0564 | 6.7129 | 0.6102 | 2.4967 | 0.99979 | ||
| Medium stress | Ads. | 0.0716 | 3.5722 | 0.8845 | 0.6433 | 0.99985 | |
| Des. | 0.0645 | 6.2429 | 0.7180 | 1.5360 | 0.99990 | ||
| High stress | Ads. | 0.0849 | 2.9787 | 0.8441 | 0.6397 | 0.99996 | |
| Des. | 0.0681 | 6.4033 | 0.5760 | 2.2986 | 0.99981 | ||
| Wood Samples | H | ΔEMCmax | RH | |
|---|---|---|---|---|
| Tissue | Environment | (Arb. Units) | (kg/kg) | (-) |
| Bark | Low stress | 0.0194 | 0.031 | 0.76 |
| Medium stress | 0.0175 | 0.030 | 0.76 | |
| High stress | 0.0189 | 0.032 | 0.71 | |
| Wood | Low stress | 0.0179 | 0.025 | 0.74 |
| Medium stress | 0.0172 | 0.025 | 0.76 | |
| High stress | 0.0193 | 0.026 | 0.72 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusiak, W.; Majka, J.; Zborowska, M.; Ratajczak, I. Chemical Composition and Related Properties of Lime (Tilia cordata Mill.) Bark and Wood as Affected by Tree Growth Conditions. Materials 2022, 15, 4033. https://doi.org/10.3390/ma15114033
Kusiak W, Majka J, Zborowska M, Ratajczak I. Chemical Composition and Related Properties of Lime (Tilia cordata Mill.) Bark and Wood as Affected by Tree Growth Conditions. Materials. 2022; 15(11):4033. https://doi.org/10.3390/ma15114033
Chicago/Turabian StyleKusiak, Władysław, Jerzy Majka, Magdalena Zborowska, and Izabela Ratajczak. 2022. "Chemical Composition and Related Properties of Lime (Tilia cordata Mill.) Bark and Wood as Affected by Tree Growth Conditions" Materials 15, no. 11: 4033. https://doi.org/10.3390/ma15114033