Research on High-Value Utilization of Carbon Derived from Tobacco Waste in Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
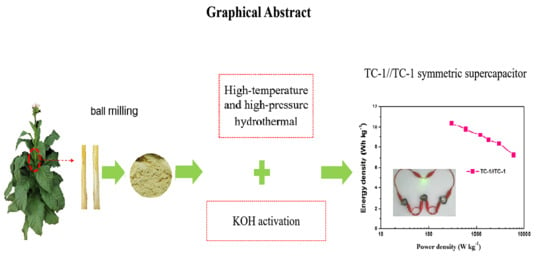
2.2. Synthesis of Tobacco Stalk-Derived Hierarchical Porous Carbon
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, C.; Hu, P.; Dong, S.; Cheng, Y.; Zhang, D.Y.; Zhang, X.H. Construction of carbon nanorods supported hydrothermal carbon and carbon fiber from waste biomass straw for high strength supercapacitor. J. Colloid Interface Sci. 2021, 582, 552–560. [Google Scholar] [CrossRef]
- Celiktas, M.S.; Alptekin, F.M. Conversion of model biomass to carbon-based material with high conductivity by using carbonization. Energy 2019, 188, 116089. [Google Scholar] [CrossRef]
- Subramani, K.; Sudhan, N.; Karnan, M.; Sathish, M. Orange peel derived activated carbon for fabrication of high-energy and high-rate supercapacitors. ChemistrySelect 2017, 2, 11384–11392. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, M.Y.; Wang, L.Q.; Huo, Y.G.; Guo, C.X.; Xin, H.Y.; Xu, S. A biomass carbon material with microtubule bundling and natural O-doping derived from goldenberry calyx and its electrochemical performance in supercapacitor. Chin. Chem. Lett. 2020, 31, 805–808. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Tran, H.N.; Chao, H.P.; Lin, C.C. Activated Carbons Derived from Teak Sawdust-Hydrochars for Efficient Removal of Methylene Blue, Copper, and Cadmium from Aqueous Solution. Water 2019, 11, 2581. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Mei, J.; Liu, G.L.; Kou, Q.; Yi, T.F.; Xiao, S.J. Nitrogen-Doped Hierarchical Porous Carbon from Wheat Straw for Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11595–11605. [Google Scholar] [CrossRef]
- Ma, Y.H.; Wang, Q.H.; Wang, X.N.; Sun, X.H.; Wang, X.Q. A comprehensive study on activated carbon prepared from spent shiitake substrate via pyrolysis with ZnCl2. J. Porous Mat. 2015, 22, 157–169. [Google Scholar] [CrossRef]
- Yaglikci, S.; Gokce, Y.; Yagmur, E.; Aktas, Z. The performance of sulphur doped activated carbon supercapacitors prepared from waste tea. Environ. Technol. 2020, 41, 36–48. [Google Scholar] [CrossRef]
- Manasa, P.; Lei, Z.J.; Ran, F. Biomass Waste Derived Low Cost Activated Carbon from Carchorus Olitorius (Jute Fiber) as Sustainable and Novel Electrode Material. J. Energy Storage 2020, 30, 101494. [Google Scholar] [CrossRef]
- Lu, C.; Qian, X.Z.; Zhu, H.Y.; Hu, Y.X.; Zhang, Y.S.; Zhang, B.M.; Kong, L.B.; Liu, M.C. 3D hierarchical porous carbon derived from direct carbonization and in-situ chemical activation of potatoes toward high-performance supercapacitors. Mater. Res. Express 2019, 6, 115615. [Google Scholar] [CrossRef]
- Li, C.; He, D.; Huang, Z.H.; Wang, M.X. Hierarchical micro-/mesoporous carbon derived from rice husk by hydrothermal pre-treatment for high performance supercapacitor. J. Electrochem. Soc. 2018, 165, A3334. [Google Scholar] [CrossRef]
- Babel, K.; Jurewicz, K. KOH activated carbon fabrics as supercapacitor material. J. Phys. Chem. Solids 2004, 65, 275–280. [Google Scholar] [CrossRef]
- Heimböckel, R.; Kraas, S.; Hoffmann, F.; Fröba, M. Increase of porosity by combining semi-carbonization and KOH activation of formaldehyde resins to prepare high surface area carbons for supercapacitor applications. Appl. Surf. Sci. 2018, 427, 1055–1064. [Google Scholar] [CrossRef]
- Wu, J.; Xia, M.; Zhang, X.; Chen, Y.; Sun, F.; Wang, X.; Yang, H.; Chen, H. Hierarchical porous carbon derived from wood tar using crab as the template: Performance on supercapacitor. J. Power Sources 2020, 455, 227982. [Google Scholar] [CrossRef]
- Zheng, L.H.; Chen, M.H.; Liang, S.X.; Lu, Q.F. Oxygen-rich hierarchical porous carbon derived from biomass waste-kapok flower for supercapacitor electrode. Diam. Relat. Mat. 2021, 113, 108267. [Google Scholar] [CrossRef]
- Li, J.M.; Wei, L.S.; Jiang, Q.M.; Liu, C.F.; Zhong, L.X.; Wang, X.Q. Salt-template assisted synthesis of cornstalk derived hierarchical porous carbon with excellent supercapacitance. Ind. Crop. Prod. 2020, 154, 112666. [Google Scholar] [CrossRef]
- Kasturi, P.R.; Harivignesh, R.; Lee, Y.S.; Ramakrishnan, K.S. Hydrothermally derived porous carbon and its improved electrochemical performance for supercapacitors using redox additive electrolytes. J. Phys. Chem. Solids 2020, 143, 109447. [Google Scholar] [CrossRef]
- Alcaraz, L.; Adán-Más, A.; Arévalo-Cid, P.; Montemor, M.F.; López, F.A. Activated carbons from winemaking biowastes for electrochemical double-layer capacitors. Front. Chem. 2020, 8, 686. [Google Scholar] [CrossRef]
- Zhou, M.; Pu, F.; Wang, Z.; Guan, S.Y. Nitrogen-doped porous carbons through KOH activation with superior performance in supercapacitors. Carbon 2014, 68, 185–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.L.; Xu, X.; Shen, H.J.; Kong, X.D.; Xu, H.M. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Teğin, Ş.Ö.; Şahin, Ö.; Baytar, O.; Izgi, M.S. Preparation and characterization of activated carbon from almond shell by microwave-assisted using ZnCl2 activator. Int. J. Chem. Technol. 2020, 4, 130–137. [Google Scholar]
- Rajasekaran, S.J.; Raghavan, V. Facile synthesis of activated carbon derived from Eucalyptus globulus seed as efficient electrode material for supercapacitors. Diam. Relat. Mat. 2020, 109, 108038. [Google Scholar] [CrossRef]
- Xia, X.H.; Liu, H.B.; Shi, L.; He, Y.D. Tobacco stem-based activated carbons for high performance supercapacitors. J. Mater. Eng. Perform. 2012, 21, 1956–1961. [Google Scholar] [CrossRef]
- Xue, J.P.; Wang, D.P.; Xia, X.H.; Chen, Y.X.; Liu, H.B. Confined red phosphorus in N-Doped hierarchically porous carbon for lithium ion batteries with enhanced rate capability and cycle stability. Microporous Mesoporous Mat. 2020, 305, 110365. [Google Scholar] [CrossRef]
- Mudyawabikwa, B.; Mungondori, H.H.; Tichagwa, L.; Katwire, D.M. Methylene blue removal using a low-cost activated carbon adsorbent from tobacco stems: Kinetic and equilibrium studies. Water Sci. Technol. 2017, 75, 2390–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Q.H.; Ma, Z.Y.; Chen, J.B.; Huang, Z.R.; Fang, Z.M.; Zhang, P.; Lin, Z.D.; Cui, J.N. S-Codoped Activated Carbon Material with Ultra-High Surface Area for High-Performance Supercapacitors. Polymers 2020, 12, 1982. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Min, S.; Wang, F.X.; Zhang, Z.G. Biomass-derived three-dimensional porous carbon membrane electrode for high-performance aqueous supercapacitors: An alternative of powdery carbon materials. J. Power Sources 2020, 466, 228347. [Google Scholar] [CrossRef]
- Shen, Y.F. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenerg. 2020, 134, 105479. [Google Scholar] [CrossRef]
- Shen, J.; Qin, Y.; Zhao, J.C. Maceral contribution to pore size distribution in anthracite in the south Qinshui Basin. Energy Fuels 2019, 33, 7234–7243. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Lv, H.J.; Chen, A.B. Silicate-assisted activation of biomass towards N-doped porous carbon sheets for supercapacitors. J. Alloy. Compd. 2021, 853, 157091. [Google Scholar] [CrossRef]
- Wang, Y.G.; Shao, C.F.; Qiu, S.J.; Zhua, Y.; Qin, M.; Meng, Y.X.; Wang, Y.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; et al. Nitrogen-doped porous carbon derived from ginkgo leaves with remarkable supercapacitance performance. Diam. Relat. Mat. 2019, 98, 107475. [Google Scholar] [CrossRef]
- Qin, C.; Wang, S.R.; Wang, Z.P.; Ji, K.; Wang, S.J.; Zeng, X.Y.; Jiang, X.M.; Liu, G. Hierarchical porous carbon derived from Gardenia jasminoides Ellis flowers for high performance supercapacitor. J. Energy Storage 2021, 13, 102061. [Google Scholar] [CrossRef]
- Kang, W.W.; Lin, B.P.; Huang, G.X.; Zhang, C.X.; Yao, Y.H.; Huo, W.T.; Xu, B.; Xing, B.L. Peanut bran derived hierarchical porous carbon for supercapacitor. J. Mater. Sci. Mater. Electron. 2018, 29, 6361–6368. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Li, M.; Zhao, Z.J. Hydrothermal preparation of highly porous carbon spheres from hemp (Cannabis sativa L.) stem hemicellulose for use in energy-related applications. Ind. Crop. Prod. 2015, 65, 216–226. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, H.P.; Zhang, H.S.; Liu, Q.; Li, R.M.; Li, B.; Wang, J. Three-dimensional hierarchical and interconnected honeycomb-like porous carbon derived from pomelo peel for high performance supercapacitors. J. Solid State Chem. 2018, 257, 64–71. [Google Scholar] [CrossRef]
- Li, Z.S.; Zhang, L.; Chen, X.; Li, B.; Wang, H.Q.; Li, Q.Y. Three-dimensional graphene-like porous carbon nanosheets derived from molecular precursor for high-performance supercapacitor application. Electrochim. Acta 2019, 296, 8–17. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wu, K.; Gao, B.; Xiao, Q.Y.; Kong, J.H.; Xiong, Q.; Xiang, P.; Zhang, X.M.; Fu, J.J. Three-dimensional activated carbon recycled from rotten potatoes for high-performance supercapacitors. Waste Biomass Valorization 2016, 7, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xiang, M.; Wu, Z.L.; Hui, J.; Huang, Q.Y.; Zhang, J.; Qin, H.F. A three-dimensional carbon electrode derived from bean sprout for supercapacitors. Ionics 2020, 26, 5705–5714. [Google Scholar] [CrossRef]
- Jia, H.Y.; Wang, S.; Sun, J.W.; Yin, K.B.; Xie, X.; Sun, L.T. Nitrogen-doped microporous carbon derived from a biomass waste-metasequoia cone for electrochemical capacitors. J. Alloys Compd. 2019, 794, 163–170. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.L.; Duan, G.G.; He, S.J.; Zhang, L.; Zhang, G.Y.; Zhou, Z.P.; Jiang, S.H. N-doped honeycomb-like porous carbon towards high-performance supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, F.; Sun, L.X.; Wu, Y.; Xia, Y.P.; Cai, X.R.; Zhong, N.K.; Zhang, H.Z.; Liu, B.; Chu, H.L. Facile method for preparation of porous carbon derived from biomass for high performance supercapacitors. Int. J. Electrochem. Sci 2019, 14, 11199–11211. [Google Scholar] [CrossRef]
- Zhang, D.; Xue, Y.C.; Chen, J.L.; Guo, X.M.; Yang, D.D.; Wang, J.C.; Zhang, J.H.; Zhang, F.; Yuan, A.H. N, S, O self-doped porous carbon nanoarchitectonics derived from pinecone with outstanding supercapacitance performances. J. Nanosci. Nanotechnol. 2020, 20, 2728–2735. [Google Scholar] [CrossRef]
- Le, P.A.; Nguyen, V.T.; Sahoo, S.K.; Tseng, T.Y.; Wei, K.H. Porous carbon materials derived from areca palm leaves for high performance symmetrical solid-state supercapacitors. J. Mater. Sci. 2020, 55, 10751–10764. [Google Scholar] [CrossRef]
- Fu, H.H.; Chen, L.; Gao, H.J.; Yu, X.K.; Hou, J.; Wang, G.; Yu, F.Y.; Li, H.Q.; Fan, C.C.; Shi, Y.L.; et al. Walnut shell-derived hierarchical porous carbon with high performances for electrocatalytic hydrogen evolution and symmetry supercapacitors. Int. J. Hydrog. Energy 2019, 45, 443–451. [Google Scholar] [CrossRef]
- Meng, S.J.; Mo, Z.L.; Li, Z.L.; Guo, R.B.; Liu, N.G. Oxygen-rich porous carbons derived from alfalfa flowers for high performance supercapacitors. Mater. Chem. Phys. 2020, 246, 112830. [Google Scholar] [CrossRef]
- Ding, F.F.; Li, J.; Du, H.M.; Zhao, J.S.; Qu, K.G.; Li, Y.W.; Zhang, X.X.; Zhang, Y.; Qin, Y.; Lu, W.Y. Highly Porous Heteroatom Doped-Carbon Derived from Orange Peel as Electrode Materials for High-Performance Supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 5632–5649. [Google Scholar] [CrossRef]
- Jeżowski, P.; Kowalczewski, P.L. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef] [Green Version]







| Abbreviations | HC: KOH (wt.%) |
| TC-0 | 1:0 |
| TC-0.5 | 1:0.5 |
| TC-1 | 1:1 |
| TC-2 | 1:2 |
| Blank-TC | No hydrothermal and activation |
| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | C (wt.%) | O (wt.%) |
|---|---|---|---|---|---|
| TC-0 | 25.3 | 0.04 | 8.05 | 95.28 | 4.72 |
| TC-0.5 | 892.4 | 0.11 | 4.38 | 92.55 | 7.45 |
| TC-1 | 1875.5 | 0.25 | 2.89 | 91.64 | 8.31 |
| TC-2 | 2270.6 | 0.21 | 2.62 | 91.10 | 8.90 |
|
Carbon Precursor |
Pre-Carbonization Condition | Single Electrode | Symmetrical Supercapacitor | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
|
Carbon/ KOH | Current Density (A g−1) | Specific Capacitance(F g−1) | Potential Window (V) | Electrolyte | Power Density (W kg−1) | Energy Density (Wh kg−1) | |||
| Potatoes | HTC: 200 °C, -MPa | 1:1 | 1 | 269 | 0–0.8 | 6M KOH | 405.60 | 4.27 | [38] |
| Bean sprout | Pyrolysis: 400 °C | 1:4 | 1 | 421 | 0–0.8 | PVA/KOH | 300 | 5.44 | [39] |
| Metasequoia cone | Pyrolysis: 500 °C | 1:3 | 0.5 | 326 | 0–1 | 6M KOH | 129 | 7.6 | [40] |
| Grape | HTC: 300 °C, -MPa | 1:8 | 0.5 | 214 | 0–1.5 | 6M KOH | 4980 | 12.6 | [41] |
| Fish seed | Pyrolysis: 500 °C | 1:3 | 1 | 306 | 0–1.0 | 6M KOH | 125 | 8.68 | [42] |
| Pinecone | -- | 5:3 | 0.5 | 285 | 0–1.0 | 6M KOH | 250 | 6.34 | [43] |
| Areca palm leaves | Pyrolysis: 600 °C | 1:2 | 0.5 | 141 | 0–1.5 | poly(vinyl alcohol)-Li2SO4 | 375 | 10.3 | [44] |
| Walnut shell | Pyrolysis: 400 °C | 1:4 | 0.5 | 262.74 | 0–1.2 | 6M KOH | 180.8 | 7.97 | [45] |
| Tobacco stalks | HTC: 280 °C, 8MPa | 1:1 | 1 | 288.4 | 0–1.2 | 6M KOH | 300 | 10.4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Qin, C.; Wang, J.; Cao, L.; Ma, Z.; Yuan, Q.; Lin, Z.; Zhang, P. Research on High-Value Utilization of Carbon Derived from Tobacco Waste in Supercapacitors. Materials 2021, 14, 1714. https://doi.org/10.3390/ma14071714
Huang Z, Qin C, Wang J, Cao L, Ma Z, Yuan Q, Lin Z, Zhang P. Research on High-Value Utilization of Carbon Derived from Tobacco Waste in Supercapacitors. Materials. 2021; 14(7):1714. https://doi.org/10.3390/ma14071714
Chicago/Turabian StyleHuang, Zhenrui, Caiyun Qin, Jun Wang, Lin Cao, Zhuwen Ma, Qinghua Yuan, Zhidan Lin, and Peng Zhang. 2021. "Research on High-Value Utilization of Carbon Derived from Tobacco Waste in Supercapacitors" Materials 14, no. 7: 1714. https://doi.org/10.3390/ma14071714