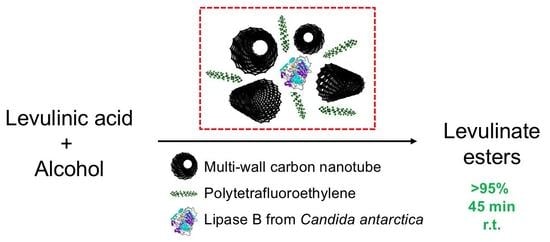
PTFE-Carbon Nanotubes and Lipase B from Candida antarctica—Long-Lasting Marriage for Ultra-Fast and Fully Selective Synthesis of Levulinate Esters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GC-FID Analyses
2.3. Thermogravimetric Analyses (TGA)
2.4. Transmission Electron Microscopy (TEM)
2.5. Raman Spectra
2.6. Synthesis of Hybrid MWCNT-PTFE Support
2.7. Immobilization of Lipases
2.8. Esterification Reaction
2.9. The Product Isolation
2.10. 1H and 13C NMR Spectra
2.11. Catalyst Recycling
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.-C.M. Continuous Flow Upgrading of Selected C2–C6 Platform Chemicals Derived from Biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef] [PubMed]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Marinas, A.; Domine, M.E. Catalytic Processes for Biomass-Derived Platform Molecules Valorisation. Top. Catal. 2020, 63, 1–20. [Google Scholar] [CrossRef]
- Song, D.; An, S.; Lu, B.; Guo, Y.; Leng, J. Arylsulfonic acid functionalized hollow mesoporous carbon spheres for efficient conversion of levulinic acid or furfuryl alcohol to ethyl levulinate. Appl. Catal. B Environ. 2015, 179, 445–457. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Song, L. Efficient and selective alcoholysis of furfuryl alcohol to alkyl levulinates catalyzed by double SO3H-functionalized ionic liquids. Green Chem. 2014, 16, 1436–1443. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Ciptonugroho, W.; Holzhäuser, F.J.; Mensah, J.B.; Hausoul, P.J.C.; Palkovits, R. Catalytic upgrading of α-angelica lactone to levulinic acid esters under mild conditions over heterogeneous catalysts. Catal. Sci. Technol. 2015, 5, 5168–5173. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Al-Shaal, M.G.; Ciptonugroho, W.; Delidovich, I.; Wang, X.; Palkovits, R.; Mohammad, G.A.S.; Wirawan, C. Synthesis of Butyl Levulinate Based on α-Angelica Lactone in the Presence of Easily Separable Heteropoly Acid Catalysts. ChemSusChem 2017, 10, 1494–1500. [Google Scholar] [CrossRef]
- Latos, P.; Szelwicka, A.; Boncel, S.; Jurczyk, S.; Swadzba-Kwasny, M.; Chrobok, A. Highly Efficient Synthesis of Alkyl Levulinates from α-Angelica Lactone, Catalyzed with Lewis Acidic Trifloaluminate Ionic Liquids Supported on Carbon Nanotubes. ACS Sustain. Chem. Eng. 2019, 7, 5184–5191. [Google Scholar] [CrossRef]
- Szelwicka, A.; Kolanowska, A.; Latos, P.; Jurczyk, S.; Boncel, S.; Chrobok, A. Carbon nanotube/PTFE as a hybrid platform for lipase B from Candida antarctica in transformation of α-angelica lactone into alkyl levulinates. Catal. Sci. Technol. 2020, 10, 3255–3264. [Google Scholar] [CrossRef]
- Melero, J.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernández, B.; Penedo, S. Efficient conversion of levulinic acid into alkyl levulinates catalyzed by sulfonic mesostructured silicas. Appl. Catal. A Gen. 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Fallavena, L.P.; Antunes, F.H.F.; Alves, J.S.; Paludo, N.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei. RSC Adv. 2014, 4, 8675–8681. [Google Scholar] [CrossRef] [Green Version]
- De Lima, L.N.; Mendes, A.A.; Fernandez-Lafuente, R.; Tardioli, P.W.; Giordano, R.D.L.C. Performance of Different Immobilized Lipases in the Syntheses of Short- and Long-Chain Carboxylic Acid Esters by Esterification Reactions in Organic Media. Molecules 2018, 23, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, J.S.; Garcia-Galan, C.; Schein, M.F.; Silva, A.M.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combined Effects of Ultrasound and Immobilization Protocol on Butyl Acetate Synthesis Catalyzed by CALB. Molecules 2014, 19, 9562–9576. [Google Scholar] [CrossRef] [Green Version]
- Marsden, S.R.; Mestrom, L.; McMillan, D.G.G.; Hanefeld, U. Thermodynamically and Kinetically Controlled Reactions in Biocatalysis—From Concepts to Perspectives. ChemCatChem 2019, 12, 426–437. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransformation 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [Green Version]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A Perspective on Continuous Flow Chemistry in the Pharmaceutical Industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. ChemInform Abstract: Lipases: Interfacial Enzymes with Attractive Applications. Angew. Chem. Int. Ed. 2010, 29, 1608–1633. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nat. Cell Biol. 1991, 351, 491–494. [Google Scholar] [CrossRef]
- Grochulski, P.; Li, Y.; Schrag, J.D.; Bouthillier, F.; Smith, P.; Harrison, D.; Rubin, B.; Cygler, M. Insights into interfacial activation from an open structure of Candida rugosa lipase. J. Biol. Chem. 1993, 268, 12843–12847. [Google Scholar] [CrossRef]
- Martinelle, M.; Holmquist, M.; Hult, K. On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1995, 1258, 272–276. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General Trend of Lipase to Self-Assemble Giving Bimolecular Aggregates Greatly Modifies the Enzyme Functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisan, J.M.; Fernandez-Lafuente, R. Self-assembly of Pseudo-monas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef]
- Wilson, L.; Palomo, J.M.; Fernandez-Lorente, G.; Illanes, A.; Guisán, J.M.; Fernandez-Lafuente, R. Effect of lipase–lipase interactions in the activity, stability and specificity of a lipase from Alcaligenes sp. Enzym. Microb. Technol. 2006, 39, 259–264. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization of Eversa Lipase on Octyl Agarose Beads and Preliminary Characterization of Stability and Activity Features. Catalysts 2018, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization on octyl-agarose beads and some catalytic features of commercial preparations of lipase a from Candida antarctica (Novocor ADL): Comparison with immobilized lipase B from Candida antarctica. Biotechnol. Prog. 2019, 35, e2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, G.D.; Borkar, I.V. Kinetic modeling of immobilized lipase catalysis in synthesis of n-butyl levulinate. Ind. Eng. Chem. Res. 2008, 47, 3358–3363. [Google Scholar] [CrossRef]
- Zhou, L.; He, Y.; Ma, L.; Jiang, Y.; Huang, Z.; Yin, L.; Gao, J. Conversion of levulinic acid into alkyl levulinates: Using lipase immobilized on meso-molding three-dimensional macroporous organosilica as catalyst. Bioresour. Technol. 2018, 247, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wang, L.; Zhou, L.; Huang, Z.; Ma, L.; He, Y.; Shi, L.; Gao, J. Virus-like organosilica nanoparticles for lipase immobilization: Characterization and biocatalytic applications. Biochem. Eng. J. 2019, 144, 125–134. [Google Scholar] [CrossRef]
- Mesbah, N.M. Covalent immobilization of a halophilic, alkalithermostable lipase LipR2 on Florisil® nanoparticles for production of alkyl levulinates. Arch. Biochem. Biophys. 2019, 667, 22–29. [Google Scholar] [CrossRef]
- Markiton, M.; Boncel, S.; Janas, D.; Chrobok, A. Highly Active Nanobiocatalyst from Lipase Noncovalently Immobilized on Multiwalled Carbon Nanotubes for Baeyer–Villiger Synthesis of Lactones. ACS Sustain. Chem. Eng. 2016, 5, 1685–1691. [Google Scholar] [CrossRef]
- Szelwicka, A.; Zawadzki, P.; Sitko, M.; Boncel, S.; Czardybon, W.; Chrobok, A. Continuous Flow Chemo-Enzymatic Baeyer–Villiger Oxidation with Superactive and Extra-Stable Enzyme/Carbon Nanotube Catalyst: An Efficient Upgrade from Batch to Flow. Org. Process. Res. Dev. 2019, 23, 1386–1395. [Google Scholar] [CrossRef]
- Szelwicka, A.; Boncel, S.; Jurczyk, S.; Chrobok, A. Exceptionally active and reusable nanobiocatalyst comprising lipase non-covalently immobilized on multi-wall carbon nanotubes for the synthesis of diester plasticizers. Appl. Catal. A Gen. 2019, 574, 41–47. [Google Scholar] [CrossRef]
- Gupta, M.N.; Kaloti, M.; Kapoor, M.; Solanki, K. Nanomaterials as Matrices for Enzyme Immobilization. Artif. Cells Blood Substit. Biotechnol. 2010, 39, 98–109. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wamer, W.; Xia, Q.; Yin, J.-J.; Fu, P.P. Enzyme-Like Activity of Nanomaterials. J. Environ. Sci. Health Part C 2014, 32, 186–211. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Abdelmaged, S.M.; Nada, A.A.; Zahran, F.; El-Wahab, S.A.; Yahea, D.; Hussein, G.; Atrees, M. Magnetic graphene based nanocomposite for uranium scavenging. J. Hazard. Mater. 2017, 322, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nat. Cell Biol. 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Mangiagalli, M.; Carvalho, H.; Natalello, A.; Ferrario, V.; Pennati, M.L.; Barbiroli, A.; Lotti, M.; Pleiss, J.; Brocca, S. Diverse effects of aqueous polar co-solvents on Candida antarctica lipase B. Int. J. Biol. Macromol. 2020, 150, 930–940. [Google Scholar] [CrossRef]
- Kasche, V. Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzym. Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Aldercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [Green Version]
- Hult, K.; Berglund, P. Enzyme promiscuity: Mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Arcens, D.; Grau, E.; Grelier, S.; Cramail, H.; Peruch, F. Impact of fatty acid structure on CALB-catalyzed esterification of glucose. Eur. J. Lipid Sci. Technol. 2020, 122, 1900294. [Google Scholar] [CrossRef] [Green Version]
- Drożdż, A.; Chrobok, A. Chemoenzymatic Baeyer–Villiger oxidation of 4-methylcyclohexanone via kinetic resolution of racemic carboxylic acids: Direct access to enantioenriched lactone. Chem. Commun. 2016, 52, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Wiemann, L.O.; Nieguth, R.; Eckstein, M.; Naumann, M.; Thum, O.; Ansorge-Schumacher, M.B. Composite Particles of Novozyme 435 and Silicone: Advancing Technical Applicability of Macroporous Enzyme Carriers. ChemCatChem 2009, 1, 455–462. [Google Scholar] [CrossRef]










| Support | SBET (m2/g) | Pore Volume (cm3/g) | PTFE Loading (wt.%) | CALB Loading (wt.%) | Immobilization Yield (%) |
|---|---|---|---|---|---|
| PTFE, grains | 1.6 | 0.004 | n/a | 0.7 | 0.1 |
| CheapTubes™ MWCNTs | 89.0 | 0.490 | n/a | 15.7 | 4.2 |
| MWCNT-PTFE (0.01 wt.%) | 83.1 | 0.204 | 0.06 | 16.0 | 4.3 |
| MWCNT-PTFE (0.10 wt.%) | 80.3 | 0.211 | 0.13 | 22.5 | 6.0 |
| MWCNT-PTFE (0.50 wt.%) | 86.3 | 0.223 | 0.46 | 15.9 | 4.2 |
| MWCNT-PTFE (1.00 wt.%) | 84.2 | 0.222 | 0.89 | 13.3 | 3.5 |
| MWCNT-PTFE (10.00 wt.%) | 87.4 | 0.215 | 4.24 | 12.4 | 3.3 |
| MWCNT-PTFE (20.00 wt.%) | 77.1 | 0.208 | 10.56 | 11.7 | 3.1 |
| Support | Lipase Loading (wt. %) a | Total Activity (U∙L) b | Specific Activity (U∙L∙kg−1) b |
|---|---|---|---|
| CALB/MWCNT-PTFE (0.10 wt.%) | 22.5 | 45,333 | 153,000 |
| CRL/MWCNT-PTFE (0.10 wt.%) | 15.6 | 13,333 | 31,200 |
| AOL/MWCNT-PTFE (0.10 wt.%) | 7.0 | 667 | 700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelwicka, A.; Siewniak, A.; Kolanowska, A.; Boncel, S.; Chrobok, A. PTFE-Carbon Nanotubes and Lipase B from Candida antarctica—Long-Lasting Marriage for Ultra-Fast and Fully Selective Synthesis of Levulinate Esters. Materials 2021, 14, 1518. https://doi.org/10.3390/ma14061518
Szelwicka A, Siewniak A, Kolanowska A, Boncel S, Chrobok A. PTFE-Carbon Nanotubes and Lipase B from Candida antarctica—Long-Lasting Marriage for Ultra-Fast and Fully Selective Synthesis of Levulinate Esters. Materials. 2021; 14(6):1518. https://doi.org/10.3390/ma14061518
Chicago/Turabian StyleSzelwicka, Anna, Agnieszka Siewniak, Anna Kolanowska, Sławomir Boncel, and Anna Chrobok. 2021. "PTFE-Carbon Nanotubes and Lipase B from Candida antarctica—Long-Lasting Marriage for Ultra-Fast and Fully Selective Synthesis of Levulinate Esters" Materials 14, no. 6: 1518. https://doi.org/10.3390/ma14061518