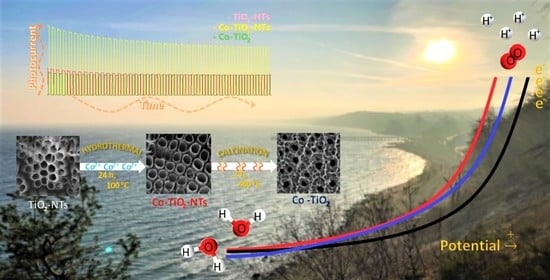
Hydrothermal Cobalt Doping of Titanium Dioxide Nanotubes towards Photoanode Activity Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals
3. Results
3.1. Preparation of the Electrode Materials
3.2. Morphology and Composition of the Samples
Scanning Electron Microscope and Energy Dispersive X-Ray Analysis
3.3. Structure
3.3.1. X-Ray Powder Diffraction
3.3.2. Raman Spectroscopy
3.3.3. X-ray Photoelectron Spectroscopy
3.4. Reflectance UV-Vis Spectroscopy
3.5. Electrochemical and Photoelectrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Cao, X.; Zhu, L.; Zheng, M. Synthesis of Functional Nanomaterials for Electrochemical Energy Storage; Springer: Singapore, 2020. [Google Scholar]
- Li-Oakey, K.D. Nanoporous Materials for Molecule Separation and Conversion; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–386. [Google Scholar]
- Hi-Gang, C.; Guang, H.; Lei, Y.; Lina, C.; Jin, Z. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. Mater. Int. 2012, 22, 535–549. [Google Scholar]
- Graetzel, M. Photoelectrochemical cells. Nature 2001, 44, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Opra, D.P.; Gnedenkov, S.V.; Sokolov, A.A.; Podgorbunsky, A.B.; Ustinov, A.Y.; Mayorov, V.Y.; Kuryavyi, V.G.; Sinebryukhov, S.L. Vanadium-doped TiO2-B/anatase mesoporous nanotubes with improved rate and cycle performance for rechargeable lithium and sodium batteries. J. Mater. Sci. Technol. 2020, 54, 181–189. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Podgorbunsky, A.B.; Sokolov, A.A.; Ustinov, A.Y.; Kuryavyi, V.G.; Mayorov, V.Y.; Zheleznov, V.V. Doping of titania with manganese for improving cycling and rate performances in lithium-ion batteries. Chem. Phys. 2020, 538, 110864. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Amal, R.; Ng, Y.H. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4. J. Mater. Chem. A 2017, 5, 16498–16521. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, P.; Yu, J. Recent advances in visible light Bi-based photocatalysts. Chin. J. Catal. 2014, 35, 989–1007. [Google Scholar]
- Mehraj, O.; Pirzada, B.M.; Mir, N.A.; Khan, M.Z.; Sabir, S. A highly efficient visible-light-driven novel pn junction Fe2O3/BiOI photocatalyst: Surface decoration of BiOI nanosheets with Fe2O3 nanoparticles. Appl. Surf. Sci. 2016, 387, 642–651. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Lisowska-Oleksiak, A.; Szybowska, K.; Jasulajtiene, V. Preparation and characterisation of visible light responsive iodine doped TiO2 electrodes. Electrochim. Acta 2010, 55, 5881–5885. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium dioxide: An over view of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Ryl, J.; Nowaczyk, G.; Zielinska, A. Morphology, Photocatalytic and Antimicrobial Properties of TiO2 Modified with Mono- and Bimetallic Copper, Platinum and Silver Nanoparticles. Nanomaterials 2019, 9, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macyk, W.; Szaciłowski, K.; Stochel, G.; Buchalska, M.; Kuncewicz, J.; Łabuz, P. Titanium (IV) complexes as direct TiO2 photosensitizers. Coord. Chem. Rev. 2010, 254, 687–2701. [Google Scholar] [CrossRef]
- Assefpour-Dezfuly, M.; Vlachos, C.; Andrews, E.H. Oxide morphology and adhesive bonding on titanium surfaces. J. Mater. Sci. 1984, 19, 3626–3639. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Canales, J.; García, R.; Bruce, P.G. Lithium-Ion Intercalation into TiO2-B Nanowires. Adv. Mater. 2005, 17, 862–865. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Macák, M.; Tsuchiya, H.; Schmuki, P. High-Aspect-Ratio TiO2 Nanotubes by Anodization of Titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 µm long anodic TiO2 nanotubes with hexagonal self-ordering. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 65–67. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.E.; Schmuki, P. Critical factors in the anodic formation of extremely ordered titania nanocavities. J. Electrochem. Soc. 2019, 166, C3389–C3398. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Jarosz, M.; Grudzien, J.; Kapusta-Kołodziej, J.; Chudecka, A.; Sołtys, M.; Sulka, G.D. Anodization of titanium alloys for biomedical applications. In Nanostructured Anodic Metal Oxides: Synthesis and Applications; Elsevier: Amsterdam, The Netherlands, 2020; p. 211. [Google Scholar]
- Li, H.H.; Wu, X.Y.; Yin, S.; Katsumata, K.; Wang, Y.H. Effect of rutile TiO2 on the photocatalytic performance of g-C3N4/brookite-TiO2-xNy photocatalyst for NO decomposition. Appl. Surf. Sci. 2017, 392, 531–539. [Google Scholar] [CrossRef]
- Szkoda, M.; Trzciński, K.; Nowak, A.P.; Coy, E.; Wicikowski, L.; Łapiński, M.; Siuzdak, K.; Lisowska-Oleksiak, A. Titania nanotubes modified by a pyrolyzed metal-organic framework with zero valent iron centers as a photoanode with enhanced photoelectrochemical, photocatalytical activity and high capacitance. Electrochim. Acta 2018, 278, 13–24. [Google Scholar] [CrossRef]
- Paulose, M.; Mor, G.K.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Visible light photoelectrochemical and water-photoelectrolysis properties of titania nanotube arrays. J. Photochem. Photobiol. A Chem. 2006, 178, 8–15. [Google Scholar] [CrossRef]
- Schulte, K.L.; DeSario, P.A.; Gray, K.A. Effect of crystal phase composition on the reductive and oxidative abilities of TiO2 nanotubes under UV and visible light. Appl. Catal. B Environ. 2010, 97, 354–360. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Shankar, K.; Mor, G.K.; Grimes, C.A. Water-Photolysis Properties of Micron-Length Highly-Ordered Titania Nanotube-Arrays. J. Nanosci. Nanotechnol. 2005, 5, 1158–1165. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Szkoda, M.; Siuzdak, K.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Facile preparation of extremely photoactive boron-doped TiO2 nanotubes arrays. Electrochem. Commun. 2016, 60, 212–215. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Sekino, T.; Okamoto, T.; Kasuga, T.; Kusunose, T.; Nakayama, T.; Niihara, K. Synthesis and Properties of Titania Nanotube Doped with Small Amount of Cations. KEM 2006, 317–318, 251–254. [Google Scholar] [CrossRef]
- Sołtys-Mróz, M.; Syrek, K.; Pierzchała, J.; Wiercigroch, E.; Malek, K.; Sulka, G.D. Band gap engineering of nanotubular Fe2O3-TiO2 photoanodes by wet impregnation. Appl. Surf. Sci. 2020, 517, 146195. [Google Scholar] [CrossRef]
- Qarechalloo, S.; Naseri, N.; Salehi, F.; Moshfegh, A.Z. Simply tuned and sustainable cobalt oxide decorated titania nanotubes for photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 464, 68–77. [Google Scholar] [CrossRef]
- Jiang, P.; Xiang, W.; Kuang, J.; Liu, W.; Cao, W. Effect of cobalt doping on the electronic, optical and photocatalytic properties of TiO2. Solid State Sci. 2015, 46, 27–32. [Google Scholar] [CrossRef]
- Monazzam, P.; Kisomi, B.F. Co/TiO2 nanoparticles: Preparation, characterization and its application for photocatalytic degradation of methylene blue. Desalination Water Treat. 2017, 63, 283–292. [Google Scholar]
- Preethi, T.; Abarna, B.; Vidhya, K.N.; Rajarajeswari, G.R. Sol–gel derived cobalt doped nano-titania photocatalytic system for solar light induced degradation of crystal violet. Ceram. Int. 2014, 40, 13159–13167. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Available online: https://www.thermofisher.com/order/catalog/product/IQLAADGACKFAKRMAVI (accessed on 16 March 2021).
- Szkoda, M.; Siuzdak, K.; Lisowka-Oleksiak, A. Optimization of electrochemical doping approach resulting in highly photoactive iodine-doped titania nanotubes. J. Sol. State Electrochem. 2016, 20, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Fabrication of nanoporous TiO2 by electrochemical anodization. Electrochim. Acta 2010, 55, 4359–4367. [Google Scholar] [CrossRef]
- Zemann, J. Crystal structures. Acta Cryst. 1965, 18, 139. [Google Scholar] [CrossRef] [Green Version]
- Spiridonova, J.; Katerski, A.; Danilson, M.; Krichevskaya, M.; Krunks, M.; Acik, I.O. Effect of the Titanium Isopropoxide: Acetylacetone Molar Ratio on the Photocatalytic Activity of TiO2 Thin Films. Molecules 2019, 24, 4326. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Chanda, A.; Rout, K.; Vasundhara, M.; Joshi, S.R.; Singh, J. Structural and magnetic study of undoped and cobalt doped TiO2 nanoparticles. RSC Adv. 2018, 8, 10939–10947. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Swamy, S.; Muddle, B.C.; Dai, Q. Size-dependent modifications of the Raman spectrum of rutile TiO2. Appl. Phys. Lett. 2006, 89, 163118. [Google Scholar] [CrossRef]
- Bassi, A.L.; Cattaneo, D.; Russo, V.; Bottani, C.E.; Barborini, E.; Mazza, T.; Piseri, P.; Milani, P.; Ernst, F.O.; Wegner, K.; et al. Raman spectroscopy characterization of Titania nanoparticles produced by flame pyrolysis: The influence of size and stoichiometry. J. Appl. Phys. 2005, 98, 074305. [Google Scholar] [CrossRef]
- Wint, T.H.M.; Smith, M.F.; Chanlek, N.; Chen, F.; Songsiriritthigul, P. Physical origin of diminishing photocatalytic efficiency for recycled TiO2 nanotubes and Ag-loaded TiO2 nanotubes in organic aqueous solution. Catalysts 2020, 10, 737. [Google Scholar] [CrossRef]
- Cabrera-German, D.; Gomez-Sosa, G.; Herrera-Gome, A. Accurate peak fitting and subsequent quantitative composition analysis of the spectrum of Co 2p obtained with Al Kα radiation: I: Cobalt spinel. Surf. Interface Anal. 2016, 48, 252–256. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A.; Islam, A.M.; Alagarsamy, P.; Mukherjee, M. Effect of oxygen vacancy and dopant concentration on the magnetic properties of high spin Co2þ doped TiO2 nanoparticles. J. Magn. Magn. Mater. 2011, 323, 440–446. [Google Scholar] [CrossRef]
- Lin, Y.B.; Yang, Y.M.; Zhuang, B.; Huang, S.L.; Wu, L.P.; Huang, Z.G.; Zhang, F.M.; Du, Y.W. Ferromagnetism of Co-doped TiO2 films prepared by plasma enhanced chemical vapour deposition (PECVD) method. J. Phys. D Appl. Phys. 2008, 41, 195007. [Google Scholar] [CrossRef]
- Li, J.G.; Buchel, R.; Isobe, M.; Mori, T.; Ishigaki, T. Cobalt-doped TiO2 nanocrystallites: Radio-frequency thermal plasma processing, phase structure, and magnetic properties. J. Phys. Chem. C 2009, 113, 8009–8015. [Google Scholar] [CrossRef]
- Antonio, J.T.; Cortés-Jácome, M.; Orozco-Cerros, S.; Palacios, E.M.; Suárez-Parra, R.; Ángeles-Chávez, C.; Navarete, J.; López-Salinas, E. Assessing optimal photoactivity on titania nanotubes using different annealing temperatures. Appl. Catal. B Environ. 2010, 100, 47–54. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Ohtsu, N.; Masahashi, N. Structural and characteristic variation of anodic oxide on pure Ti with anodization duration. Appl. Surf. Sci. 2013, 283, 1018–1023. [Google Scholar] [CrossRef]
- Dong, G.; Hu, H.; Huang, X.; Zhang, Y.; Bi, Y. Rapid activation of Co3O4 cocatalysts with oxygen vacancies on TiO2 photoanodes for efficient water splitting. J. Mater. Chem. A 2018, 6, 21003–21009. [Google Scholar] [CrossRef]
- Szkoda, M.; Siuzdak, K.; Lisowska-Oleksiak, A. Non-metal doped TiO2 nanotube arrays for high efficiency photocatalytic decomposition of organic species in water. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 84, 141–145. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Wang, J. Electrochemical Methods: Fundamentals and Applications. J. Chem. Educ. 1983, 60, A25. [Google Scholar]
- Szkoda, M.; Trzciński, K.; Lisowska-Oleksiak, A.; Siuzdak, K. Electrochemical and photoelectrochemical properties of the interface between titania nanotubes covered by conducting polymer in aqueous—The effect of various geometry and electrolytes concentration. Appl. Surf. Sci. 2018, 448, 309–319. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y. Photoelectrochemical water splitting on chromium-doped titanium dioxide nanotube photoanodes prepared by single-step anodizing. J. Alloys Compd. 2015, 637, 393–400. [Google Scholar] [CrossRef]
- Chakhari, W.; Naceur, J.B.; Taieb, S.B.; Assaker, I.B.; Chtourou, R. Fe-doped TiO2 nanorods with enhanced electrochemical properties as ef fi cient photoanode materials. J. Alloys Compd. 2017, 708, 862–870. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Annapoorna, I.; Sumliner, J.M.; Ramakrishna, M.; Hebalkar, N.Y.; Padmanabham, G. Applied Surface Science Preparation and characterization of Cu-doped TiO2 materials for electrochemical, photoelectrochemical, and photocatalytic applications. Appl. Surf. Sci. 2014, 293, 229–247. [Google Scholar] [CrossRef]
- Guaglianoni, W.C.; Florence, C.L.; Bonatto, F.; Venturini, J.; Arcaro, S.; Alves, A.K.; Bergmann, C.P. Novel nanoarchitectured cobalt-doped TiO2 and carbon nanotube arrays: Synthesis and photocurrent performance. Ceram. Int. 2019, 45, 2439–2445. [Google Scholar] [CrossRef]
- Venturini, J.; Bonatto, F.; Guaglianoni, W.C.; Lemes, T.; Arcaro, S.; Alves, A.K.; Bergmann, C.P. Applied Surface Science Cobalt-doped titanium oxide nanotubes grown via one-step anodization for water splitting applications. Appl. Surf. Sci. 2019, 464, 351–359. [Google Scholar] [CrossRef]
- Szkoda, M.; Lisowska-Oleksiak, A.; Siuzdak, K. Optimization of boron-doping process of titania nanotubes via electrochemical method toward enhanced photoactivity. J. Solid State Electrochem. 2016, 20, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lu, D.; Zhang, Z.; Yang, J. Enhancement of visible-light-induced photocurrent and photocatalytic activity of V and N codoped TiO2 nanotube array films. J. Electrochem. Soc. 2014, 161, H416–H421. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, D.; Namdeo, A.; Kumar, A. ScienceDirect Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrog. Energy 2019, 45, 2729–2744. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe—TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef]










| Sample | D101 (nm) | D112 (nm) | D200 (nm) | D211 (nm) |
|---|---|---|---|---|
| TiO2-NTs | 18.2 | 28.2 | 16.5 | 25.5 |
| Co-TiO2-NTs | 15.3 | 41.1 | 14.6 | 17.5 |
| Co-TiO2 | 19.7 | 34.6 | 15.0 | 13.3 |
| Sample | Efb (V) for 1 Hz | Efb (V) for 100 Hz | Efb (V) for 1000 Hz |
|---|---|---|---|
| TiO2-NTs | −0.21 ± 0.02 | −0.22 ± 0.02 | −0.25 ± 0.02 |
| Co-TiO2-NTs | −0.02 ± 0.02 | 0.00 ± 0.02 | 0.00 ± 0.02 |
| Co-TiO2 | 0.15 ± 0.02 | 0.06 ± 0.02 | 0.02 ± 0.02 |
| Sample | Δj (μA·cm−2) | jl/jd | Δj/P (μA∙W−1) |
|---|---|---|---|
| TiO2-NTs | 12.51 | 33.08 | 12.5 |
| Co-TiO2-NTs | 32.71 | 137.29 | 32.7 |
| Co-TiO2 | 11.68 | 33.44 | 11.7 |
| Electrode Material | Luminous Intensity (mW·cm2) | Energy Bandgap, Eg (eV) | Photocurrent Density (μA·cm 2)/E * (V) | Enhancement Factor (jdoped/jTiO2) | Ref. |
|---|---|---|---|---|---|
| TiO2-NTs | 100 | 2.99 | 12.9 at 0.5 V | 1 | This work |
| Co-TiO2 | 2.85 | 12.0 at 0.5 V | 0.9 | ||
| Co-TiO2-NTs | 2.92 | 33.3 at 0.5 V | 2.6 | ||
| Co-TiO2-NTs | 100 | 3.09 | 95.0 at 0.5 V | 1.5 | [68] |
| Co-TiO2-NTs | 100 | no data | 40.0 at 0.4 V | 3.0 | [67] |
| Cr-TiO2-NTs | 100 | 2.82 | 360.0 at 1.0 V | 9.2 | [64] |
| B-TiO2-NTs | 100 | 2.91 | 311.0 at 0.5 V | 7.4 | [69] |
| V-TiO2-NTs | 16 | no data | 5.8 at 0.5 V | 4.8 | [70] |
| Ag-TiO2 film | 4.4 | 2.5 | 1.2 at 0.2 V | 3.5 | [71] |
| Fe-TiO2 nanorods | 100 | 3.12 | 550.0 at 0 V | 5.5 | [65] |
| Cu-TiO2 film | 44.42 | 2.82 | 18.2 at 0.4 V | 1.3 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wtulich, M.; Szkoda, M.; Gajowiec, G.; Gazda, M.; Jurak, K.; Sawczak, M.; Lisowska-Oleksiak, A. Hydrothermal Cobalt Doping of Titanium Dioxide Nanotubes towards Photoanode Activity Enhancement. Materials 2021, 14, 1507. https://doi.org/10.3390/ma14061507
Wtulich M, Szkoda M, Gajowiec G, Gazda M, Jurak K, Sawczak M, Lisowska-Oleksiak A. Hydrothermal Cobalt Doping of Titanium Dioxide Nanotubes towards Photoanode Activity Enhancement. Materials. 2021; 14(6):1507. https://doi.org/10.3390/ma14061507
Chicago/Turabian StyleWtulich, Mariusz, Mariusz Szkoda, Grzegorz Gajowiec, Maria Gazda, Kacper Jurak, Mirosław Sawczak, and Anna Lisowska-Oleksiak. 2021. "Hydrothermal Cobalt Doping of Titanium Dioxide Nanotubes towards Photoanode Activity Enhancement" Materials 14, no. 6: 1507. https://doi.org/10.3390/ma14061507