Recycling of Waste Solution after Hydrothermal Conversion of Fly Ash on a Semi-Technical Scale for Zeolite Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
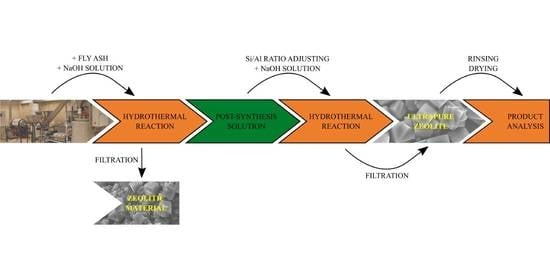
2.2. Synthesis
2.3. Characterization Methods
3. Results and Discussion
3.1. Zeolites Particle Size Distrubution
3.2. XRF Chemical Composition
3.3. XRD Mineralogy Characterization
3.4. TEM
3.5. FT-IR
3.6. ASAP 2020
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belviso, C. State-of-the-Art Applications of Fly Ash from Coal and Biomass: A Focus on Zeolite Synthesis Processes and Issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Xing, Y.; Guo, F.; Xu, M.; Gui, X.; Li, H.; Li, G.; Xia, Y.; Han, H. Separation of Unburned Carbon from Coal Fly Ash: A Review. Powder Technol. 2019, 353, 372–384. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A Comprehensive Review on the Applications of Coal Fly Ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-Derived Unburned Carbons in Fly Ash: A Review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A Review on Fly Ash Characteristics – Towards Promoting High Volume Utilization in Developing Sustainable Concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Inada, M.; Eguchi, Y.; Enomoto, N.; Hojo, J. Synthesis of Zeolite from Coal Fly Ashes with Different Silica–Alumina Composition. Fuel 2005, 84, 299–304. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A Review on the Utilization of Fly Ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Hu, T.; Gao, W.; Liu, X.; Zhang, Y.; Meng, C. Synthesis of Zeolites Na-A and Na-X from Tablet Compressed and Calcinated Coal Fly Ash. R. Soc. Open Sci. 2017, 4, 170921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of Phosphate from Water by Lanthanum-Modified Zeolites Obtained from Fly Ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef]
- Styczeń, J.; Barnat-Hunek, D.; Panek, R.; Franus, W. The Microstructural and Physical Properties of Renovation Renders with Clinoptilolite, Na-P1 and Na-X Zeolites. Constr. Build. Mater. 2020, 261, 120016. [Google Scholar] [CrossRef]
- Woszuk, A.; Zofka, A.; Bandura, L.; Franus, W. Effect of Zeolite Properties on Asphalt Foaming. Constr. Build. Mater. 2017, 139, 247–255. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic Fuel Oils Produced from the Pyrolysis-Catalysis of Polyethylene Plastic with Metal-Impregnated Zeolite Catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Le, T.T.; Chawla, A.; Rimer, J.D. Impact of Acid Site Speciation and Spatial Gradients on Zeolite Catalysis. J. Catal. 2020, 391, 56–68. [Google Scholar] [CrossRef]
- Bates, J.S.; Gounder, R. Influence of Confining Environment Polarity on Ethanol Dehydration Catalysis by Lewis Acid Zeolites. J. Catal. 2018, 365, 213–226. [Google Scholar] [CrossRef]
- Vilaça, N.; Bertão, A.R.; Prasetyanto, E.A.; Granja, S.; Costa, M.; Fernandes, R.; Figueiredo, F.; Fonseca, A.M.; De Cola, L.; Baltazar, F.; et al. Surface Functionalization of Zeolite-Based Drug Delivery Systems Enhances Their Antitumoral Activity in Vivo. Mater. Sci. Eng. C 2020, 111721. [Google Scholar] [CrossRef]
- Souza, I.M.S.; Borrego-Sánchez, A.; Sainz-Díaz, C.I.; Viseras, C.; Pergher, S.B.C. Study of Faujasite Zeolite as a Modified Delivery Carrier for Isoniazid. Mater. Sci. Eng. C 2021, 118, 111365. [Google Scholar] [CrossRef] [PubMed]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical Applications of Zeolite-Based Materials: A Review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yue, M.B.; Wang, X.N.; Qin, D. Synthesis of Nanosized TS-1 Zeolites through Solid Transformation Method with Unprecedented Low Usage of Tetrapropylammonium Hydroxide. Microporous Mesoporous Mater. 2015, 217, 96–101. [Google Scholar] [CrossRef]
- Otieno, S.O.; Kengara, F.O.; Kemmegne-Mbouguen, J.C.; Langmi, H.W.; Kowenje, C.B.O.; Mokaya, R. The Effects of Metakaolinization and Fused-Metakaolinization on Zeolites Synthesized from Quartz Rich Natural Clays. Microporous Mesoporous Mater. 2019, 290, 109668. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, Faujasite and A-Type Zeolite from 2:1dioctahedral and 2:1:1 Trioctahedral Clay Minerals. A Singular Review of Synthesis Methods through Laboratory Trials at a Low Incubation Temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, X.; Liu, T.; Liu, H.; Wang, T.; Yuan, P.; Zhu, H.; Bai, Z.; Bao, X. Template Free Synthesis of Hierarchical Porous Zeolite Beta with Natural Kaolin Clay as Alumina Source. Microporous Mesoporous Mater. 2020, 293, 109772. [Google Scholar] [CrossRef]
- Pichór, W.; Mozgawa, W.; Król, M.; Adamczyk, A. Synthesis of the Zeolites on the Lightweight Aluminosilicate Fillers. Mater. Res. Bull. 2014, 49, 210–215. [Google Scholar] [CrossRef]
- Wang, Y.; Du, T.; Jia, H.; Qiu, Z.; Song, Y. Synthesis, Characterization and CO2 Adsorption of NaA, NaX and NaZSM-5 from Rice Husk Ash. Solid State Sci. 2018, 86, 24–33. [Google Scholar] [CrossRef]
- Mukherjee, S.; Barman, S.; Halder, G. Fluoride Uptake by Zeolite NaA Synthesized from Rice Husk: Isotherm, Kinetics, Thermodynamics and Cost Estimation. Groundw. Sustain. Dev. 2018, 7, 39–47. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of Coal Fly Ash to Zeolite Utilizing Microwave and Ultrasound Energies: A Review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Wdowin, M.; Franus, W. Synthesis of Faujasite (FAU) and Tschernichite (LTA) Type Zeolites as a Potential Direction of the Development of Lime Class C Fly Ash. Int. J. Miner. Process. 2017, 166, 69–78. [Google Scholar] [CrossRef]
- Bandura, L.; Kołodyńska, D.; Franus, W. Adsorption of BTX from Aqueous Solutions by Na-P1 Zeolite Obtained from Fly Ash. Process. Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Mondragon, F.; Rincon, F.; Sierra, L.; Escobar, J.; Ramirez, J.; Fernandez, J. New Perspectives for Coal Ash Utilization: Synthesis of Zeolitic Materials. Fuel 1990, 69, 263–266. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Fernández-Turiel, J.L.; López-Soler, A. Synthesis of Zeolites by Alkaline Activation of Ferro-Aluminous Fly Ash. Fuel 1995, 74, 1226–1231. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The Conversion Technology of Fly Ash into Zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yao, B.; Xia, Y.; Huang, H.; Gan, Y.; Zhang, W. Coal Fly Ash Derived Zeolite for Highly Efficient Removal of Ni2+ Inwaste Water. Powder Technol. 2020, 367, 40–46. [Google Scholar] [CrossRef]
- Tauanov, Z.; Tsakiridis, P.E.; Mikhalovsky, S.V.; Inglezakis, V.J. Synthetic Coal Fly Ash-Derived Zeolites Doped with Silver Nanoparticles for Mercury (II) Removal from Water. J. Environ. Manag. 2018, 224, 164–171. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Q.; Wu, H. Synthesis of Zeolite-like Material by Hydrothermal and Fusion Methods Using Municipal Solid Waste Fly Ash. Procedia Environ. Sci. 2016, 31, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Ojumu, T.V.; Du Plessis, P.W.; Petrik, L.F. Synthesis of Zeolite A from Coal Fly Ash Using Ultrasonic Treatment—A Replacement for Fusion Step. Ultrason. Sonochem. 2016, 31, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Hollman, G.G.; Steenbruggen, G.; Janssen-Jurkovičová, M. A Two-Step Process for the Synthesis of Zeolites from Coal Fly Ash. Fuel 1999, 78, 1225–1230. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakai, Y.; Hino, R. Formation of Na-A and -X Zeolites from Waste Solutions in Conversion of Coal Fly Ash to Zeolites. Mater. Res. Bull. 2002, 37, 1873–1884. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 17 November 2020).
- Krachuamram, S.; Chanapattharapol, K.C.; Kamonsutthipaijit, N. Synthesis and Characterization of NaX-Type Zeolites Prepared by Different Silica and Alumina Sources and Their CO2 Adsorption Properties. Microporous Mesoporous Mater. 2021, 310, 110632. [Google Scholar] [CrossRef]
- Muriithi, G.N.; Petrik, L.F.; Doucet, F.J. Synthesis, Characterisation and CO2 Adsorption Potential of NaA and NaX Zeolites and Hydrotalcite Obtained from the Same Coal Fly Ash. J. CO2 Util. 2020, 36, 220–230. [Google Scholar] [CrossRef]
- Yurekli, Y. Determination of Adsorption Characteristics of Synthetic NaX Nanoparticles. J. Hazard. Mater. 2019, 378, 120743. [Google Scholar] [CrossRef]
- Yang, T.; Han, C.; Liu, H.; Yang, L.; Liu, D.; Tang, J.; Luo, Y. Synthesis of Na-X Zeolite from Low Aluminum Coal Fly Ash: Characterization and High Efficient As(V) Removal. Adv. Powder Technol. 2019, 30, 199–206. [Google Scholar] [CrossRef]
- Djamel, N.; Samira, A. Mechanism of Cu2+ Ions Uptake Process by Synthetic NaA Zeolite from Aqueous Solution: Characterization, Kinetic, Intra-Crystalline Diffusion and Thermodynamic Studies. J. Mol. Liq. 2020, 114642. [Google Scholar] [CrossRef]
- Ryczkowski, R.; Goscianska, J.; Panek, R.; Franus, W.; Przybysz, K.; Grams, J. Sustainable Nickel Catalyst for the Conversion of Lignocellulosic Biomass to H2-Rich Gas. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Volli, V.; Purkait, M.K. Selective Preparation of Zeolite X and A from Flyash and Its Use as Catalyst for Biodiesel Production. J. Hazard. Mater. 2015, 297, 101–111. [Google Scholar] [CrossRef]
- Sandomierski, M.; Buchwald, Z.; Koczorowski, W.; Voelkel, A. Calcium Forms of Zeolites A and X as Fillers in Dental Restorative Materials with Remineralizing Potential. Microporous Mesoporous Mater. 2020, 294, 109899. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic Zeolites from Fly Ash as Effective Mineral Sorbents for Land-Based Petroleum Spills Cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Rotko, M.; Franus, W. Synthetic Zeolites from Fly Ash for an Effective Trapping of BTX in Gas Stream. Microporous Mesoporous Mater. 2016, 223, 1–9. [Google Scholar] [CrossRef]
- Lim, W.-R.; Lee, C.-H.; Hamm, S.-Y. Synthesis and Characteristics of Na-A Zeolite from Natural Kaolin in Korea. Mater. Chem. Phys. 2021, 261, 124230. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel Zeolite Na-X Synthesized from Fly Ash as a Heterogeneous Catalyst in Biodiesel Production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Kunecki, P.; Panek, R.; Wdowin, M.; Bień, T.; Franus, W. Influence of the Fly Ash Fraction after Grinding Process on the Hydrothermal Synthesis Efficiency of Na-A, Na-P1, Na-X and Sodalite Zeolite Types. Int. J. Coal Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Król, M.; Mozgawa, W.; Jastrzębski, W.; Barczyk, K. Application of IR Spectra in the Studies of Zeolites from D4R and D6R Structural Groups. Microporous Mesoporous Mater. 2012, 156, 181–188. [Google Scholar] [CrossRef]
- Sandomierski, M.; Strzemiecka, B.; Voelkel, A. The Influence of Ion Exchange in Zeolite X on the Properties of Phenol-Formaldehyde Composites. Int. J. Adhes. Adhes. 2020, 100, 102625. [Google Scholar] [CrossRef]
- Tounsi, H.; Mseddi, S.; Djemel, S. Preparation and Characterization of Na-LTA Zeolite from Tunisian Sand and Aluminum Scrap. Phys. Procedia 2009, 2, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.; Aroujalian, A.; Raisi, A.; Dabir, B.; Fathizadeh, M. Preparation and Characterization of Nano-NaX Zeolite by Microwave Assisted Hydrothermal Method. Adv. Powder Technol. 2014, 25, 722–727. [Google Scholar] [CrossRef]
- Du, X.; Wu, E. Porosity of Microporous Zeolites A, X and ZSM-5 Studied by Small Angle X-Ray Scattering and Nitrogen Adsorption. J. Phys. Chem. Solids 2007, 68, 1692–1699. [Google Scholar] [CrossRef]








| Zeolite | NaOH Conc. (M) | VNaOH (dm3) | Al Source (g) | Vwas.sol. (dm3) | Time (h) | Temperature (°C) | Product Mass (g) |
|---|---|---|---|---|---|---|---|
| Na-X-LS | 3 | 0.1 | 0.9 | 0.1 | 24 | 70 | 3 |
| Na-X-TS | 3 | 50 | 450 | 50 | 24 | 70 | 700 |
| Na-A-LS | 3 | 0.1 | 1.3 | 0.1 | 24 | 80 | 5 |
| Na-A-TS | 3 | 50 | 660 | 50 | 24 | 80 | 1180 |
| Fraction (µm) | Na-X-LS | Na-X-TS | Na-A-LS | Na-A-TS |
|---|---|---|---|---|
| (% Volume) | ||||
| 0.01–2 | 6.26 | 11.86 | 8.22 | 13.23 |
| 2–20 | 60.97 | 67.31 | 66.09 | 70.87 |
| 20–50 | 16.00 | 7.68 | 21.86 | 4.51 |
| 50–100 | 6.68 | 4.84 | 1.94 | 3.89 |
| 100–250 | 5.33 | 4.80 | 1.32 | 5.51 |
| 250–500 | 4.08 | 2.60 | 0.58 | 1.87 |
| 500–1000 | 0.68 | 0.91 | - | 0.12 |
| 1000–2000 | - | - | - | - |
| Component | Na-X-LS | Na-X-TS | Na-A-LS | Na-A-TS |
|---|---|---|---|---|
| (%) | ||||
| Na2O | 12.42 | 6.78 | 14.69 | 10.76 |
| MgO | nd | 1.47 | nd | 0.62 |
| Al2O3 | 23.15 | 22.42 | 25.43 | 25.86 |
| SiO2 | 41.65 | 39.34 | 40.45 | 38.26 |
| K2O | 1.22 | 0.69 | 0.98 | 0.63 |
| CaO | 0.05 | 7.58 | 0.02 | 4.31 |
| TiO2 | 0.02 | 0.04 | 0.02 | 0.03 |
| Fe2O3 | 0.52 | 0.93 | 0.58 | 1.27 |
| LOI | 20.83 | 20.75 | 17.66 | 17.86 |
| Zeolite | SBET (m2/g) | Smic. (m2/g) | Smes. (m2/g) | Vmic. (cm3/g) | Vmes. (cm3/g) |
|---|---|---|---|---|---|
| Na-X-LS | 734 | 693.194 | 27.501 | 0.302 | 0.020 |
| Na-X-TS | 671 | 626.911 | 36.344 | 0.314 | 0.052 |
| Na-A-LS | 8 | 2.123 | 3.981 | 0.001 | 0.014 |
| Na-A-TS | 19 | 3.542 | 10.922 | 0.002 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panek, R.; Madej, J.; Bandura, L.; Słowik, G. Recycling of Waste Solution after Hydrothermal Conversion of Fly Ash on a Semi-Technical Scale for Zeolite Synthesis. Materials 2021, 14, 1413. https://doi.org/10.3390/ma14061413
Panek R, Madej J, Bandura L, Słowik G. Recycling of Waste Solution after Hydrothermal Conversion of Fly Ash on a Semi-Technical Scale for Zeolite Synthesis. Materials. 2021; 14(6):1413. https://doi.org/10.3390/ma14061413
Chicago/Turabian StylePanek, Rafał, Jarosław Madej, Lidia Bandura, and Grzegorz Słowik. 2021. "Recycling of Waste Solution after Hydrothermal Conversion of Fly Ash on a Semi-Technical Scale for Zeolite Synthesis" Materials 14, no. 6: 1413. https://doi.org/10.3390/ma14061413