GAKTpore: Stereological Characterisation Methods for Porous Foams in Biomedical Applications
Abstract
:1. Introduction
2. GAKTpore Algorithm and Metrics
2.1. Image Segmentation
2.2. Contour Determination, Porosity and Pore Area
2.3. Segment Banding
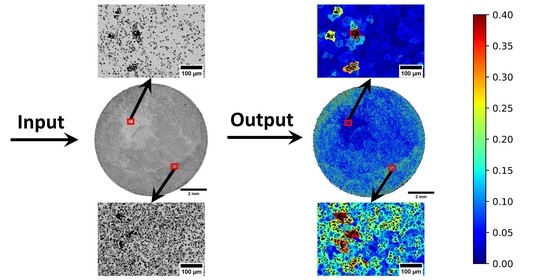
2.4. Pore Dispersion/Homogeneity Determination
2.5. Calculation of Max Radius-Largest Sphere Fitting Through a Pore (LSTP)
2.6. Calculation of Shape Factors
3. Material Fabrication and Imaging
3.1. Digital Synthetic Features for Validation
3.2. Fabrication of Foams and Scaffolds
3.3. Imaging
3.4. Manual Measurement
4. Results
4.1. GAKTpore Validation on Synthetic Images
4.1.1. Validation of LSTP
4.1.2. Validation of Shape Factors
4.2. GAKTpore Validation on SEM Micrographs
4.2.1. TEM Athene 75 Mesh Standard Copper Grid (G210)
4.2.2. Micrographs of Sintered Samples
4.2.3. GAKTpore Shape Factor and Pore Dispersion Statistics
4.3. Tissue Engineering Application
5. Discussion
5.1. Algorithm
5.2. Applications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faraj, K.A.; Van Kuppevelt, T.H.; Daamen, W.F. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007, 13, 2387–2394. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.J.; Tria, A.J.; Zawadsky, J.P.; Kato, Y.P.; Christiansen, D.L.; Silver, F.H. Development of a reconstituted collagen tendon prosthesis. A preliminary implantation study. J. Bone Joint Surg. 1989, 71, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, Y.J.; Cho, H.J.; Shin, H. Guidance of In Vitro Migration of Human Mesenchymal Stem Cells and In Vivo Guided Bone Regeneration Using Aligned Electrospun Fibers. Tissue Eng. Part A 2014, 20, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, C.J.; Mehr, M.; Buxton, P.G.; Best, S.M.; Cameron, R.E. Cell Invasion in Collagen Scaffold Architectures Characterized by Percolation Theory. Adv. Healthc. Mater. 2015, 4, 1317–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Collagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef]
- Ashby, M.F. (Ed.) Chapter 4—Properties of Metal Foams. In Metal Foams; Butterworth-Heinemann: Burlington, VT, USA, 2000; pp. 40–54. [Google Scholar]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wałowski, G. Assessment of gas permeability coefficient of porous materials. J. Sustain. Min. 2017, 16, 55–65. [Google Scholar] [CrossRef]
- Heikkinen, M.S.A.; Harley, N.H. Experimental Investigation of Sintered Porous Metal Filters. J. Aerosol Sci. 2000, 31, 721–738. [Google Scholar] [CrossRef]
- Pałka, K.; Adamek, G.; Jakubowicz, J. Microstructure and Interconnections Characteristics of Titanium Foam. Key Eng. Mater. 2016, 687, 25–32. [Google Scholar] [CrossRef]
- Suzuki, T.; Yasuda, Y.; Terasaki, T.; Morita, T.; Kawana, Y.; Ishikawa, D.; Nishimura, M.; Nakako, H.; Kurafuchi, K. Macro- and Micro-Deformation Behavior of Sintered-Copper Die-Attach Material. IEEE Trans. Device Mater. Reliab. 2018, 18, 54–63. [Google Scholar] [CrossRef]
- Parvanian, A.M.; Panjepour, M. Mechanical behavior improvement of open-pore copper foams synthesized through space holder technique. Mater. Des. 2013, 49, 834–841. [Google Scholar] [CrossRef]
- Waters, C.; Ajinola, S.; Salih, M. Dissolution Sintering Technique to Create Porous Copper with Sodium Chloride Using Polyvinyl Alcohol Solution through Powder Metallurgy. Am. J. Eng. Appl. Sci. 2016, 9, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Salimon, A.; Brechet, Y.; Ashby, M.F.; Greer, A.L. Potential applications for steel and titanium metal foams. J. Mater. Sci. 2005, 40, 5793–5799. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fung, T.; Zhang, L.P.; Zhang, F.L. Lost carbonate sintering process for manufacturing metal foams. Scr. Mater. 2005, 52, 295–298. [Google Scholar] [CrossRef]
- Spoerke, D.E.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A bioactive titanium foam scaffold for bone repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef]
- Thelen, S.; Barthelat, F.; Brinson, L.C. Mechanics considerations for microporous titanium as an orthopedic implant material. J. Biomed. Mater. Res. Part A 2004, 69A, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Lee, P.D.; Lindley, T.C.; Dashwood, R.J.; Ferrie, E.; Imwinkelried, T. Characterization of the structure and permeability of titanium foams for spinal fusion devices. Acta Biomater. 2009, 5, 477–487. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Morrison, S.J.; Klawitter, J.J. Tissue reaction to three ceramics of porous and non-porous structures. J. Biomed. Mater. Res. 1972, 6, 347–374. [Google Scholar] [CrossRef]
- Sepulveda, P.; Bressiani, A.H.; Bressiani, J.C.; Meseguer, L.; König, B. In vivo evaluation of hydroxyapatite foams. J. Biomed. Mater. Res. 2002, 62, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Braem, A.; Chaudhari, A.; Cardoso, M.V.; Schrooten, J.; Duyck, J.; Vleugels, J. Peri- and intra-implant bone response to microporous Ti coatings with surface modification. Acta Biomater. 2014, 10, 986–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siti Athirah, R.; Mazlan, M.; Amalina, N.; Jumahat, A.; Ismail, M.H. Processing of Porous Copper by Powder Metallurgy Route with Different Types of Space Holder Materials (SHMs). Adv. Mater. Res. 2015, 1113, 110–115. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; McLaughlin, J.; Bonallo, R. Effect of Pore Size, Morphology and Orientation on the Bulk Stiffness of a Porous Ti35Nb4Sn Alloy. J. Mater. Eng. Perform. 2018, 27, 2899–2909. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Ren, W.; Li, G.; Yang, R.; Wang, T. A Theoretical Analysis of Pore Size Distribution Effects on Shale Apparent Permeability. Geofluids 2017, 2017, 7492328. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wang, Z.; Zhao, N. Effect of pore size and relative density on the mechanical properties of open cell aluminum foams. Scr. Mater. 2007, 56, 169–172. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- Harley, B.A.C.; Gibson, L.J. In vivo and in vitro applications of collagen-GAG scaffolds. Chem. Eng. J. 2008, 137, 102–121. [Google Scholar] [CrossRef]
- Copes, F. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; Harley, B.A.; So, P.T.C. Biologically active collagen-based scaffolds: Advances in processing and characterization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2123–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. A design protocol for tailoring ice-templated scaffold structure. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Barozzi, M.; Sciancalepore, C.; Galetti, M.; Caffarra, C.; Lagonegro, P.; Scavia, G.; et al. Highly-defined bioprinting of long-term vascularized scaffolds with Bio-Trap: Complex. geometry functionalization and process parameters with computer aided tissue engineering. Materialia 2020, 9, 100560. [Google Scholar] [CrossRef]
- Ishizaki, K.; Komarneni, S.; Nanko, M. Porous Materials; Process Technology and Applications; Springer: New York, NY, USA, 1998. [Google Scholar]
- Nenchev, B.; Strickland, J.; Tassenberg, K.; Perry, S.; Gill, S.; Dong, H. Automatic Recognition of Dendritic Solidification Structures: DenMap. J. Imaging 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.; Nenchev, B.; Perry, S.; Tassenberg, K.; Gill, S.; Panwisawas, C.; Dong, H.; D’Souza, N.; Irwin, S. On the nature of hexagonality within the solidification structure of single crystal alloys: Mechanisms and applications. Acta Materialia 2020, 200, 417–431. [Google Scholar] [CrossRef]
- Grove, C.; Jerram, D.A. jPOR: An ImageJ macro to quantify total optical porosity from blue-stained thin sections. Comput. Geosci. 2011, 37, 1850–1859. [Google Scholar] [CrossRef]
- Sauzet, O.; Cammas, C.; Gilliot, J.M.; Bajard, M.; Montagne, D. Development of a novel image analysis procedure to quantify biological porosity and illuvial clay in large soil thin sections. Geoderma 2017, 292, 135–148. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [Green Version]
- Hotaling, N.A. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-S.; Lu, X.-M.; Lu, Q.-H. Effects of concave and convex substrate curvature on cell mechanics and the cytoskeleton. Chin. Chem. Lett. 2017, 28, 818–826. [Google Scholar] [CrossRef]
- Kim, M.-H.; Sawada, Y.; Taya, M.; Kino-oka, M. Influence of surface topography on the human epithelial cell response to micropatterned substrates with convex and concave architectures. J. Biol. Eng. 2014, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.; van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W.C. Geometry as a Factor for Tissue Growth: Towards Shape Optimization of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bradski, G. The OpenCV Library. Dr. Dobb’s J. Softw. Tools 2000, 25, 120–125. [Google Scholar]
- Friel, J.J. Practical Guide to Image Analysis; ASM International: Cleveland, OH, USA, 2000. [Google Scholar]
- Cah, R.W. Quantitative image analysis of microstructures. Edited by H. E. Exner and H. P. Hougardy. DGM Informations- gesellschaft Verlag, Oberursel, 1988. 235 pp., bound, DM 95, $ 68.—ISBN 3-88355-132-5 (English Edition). Adv. Mater. 1990, 2, 111–112. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Howard, D.; Waller, A.K.; Foster, H.R.; Mueller, A.; Moreau, T.; Evans, A.L.; Arumugam, M.; Chalon, G.B.; Vriend, E.; et al. Structurally graduated collagen scaffolds applied to the ex vivo generation of platelets from human pluripotent stem cell-derived megakaryocytes: Enhancing production and purity. Biomaterials 2018, 182, 135–144. [Google Scholar] [CrossRef]
- Nair, M.; Shepherd, J.H.; Best, S.M.; Cameron, R.E. MicroCT analysis of connectivity in porous structures: Optimizing data acquisition and analytical methods in the context of tissue engineering. J. R. Soc. Interface 2020, 17, 20190833. [Google Scholar] [CrossRef] [PubMed]
- Arifvianto, B.; Leeflang, M.A.; Zhou, J. Characterization of the porous structures of the green body and sintered biomedical titanium scaffolds with micro-computed tomography. Mater. Charact. 2016, 121, 48–60. [Google Scholar] [CrossRef]
- Muñoz, S.; Pavón, J.; Rodríguez-Ortiz, J.A.; Civantos, A.; Allain, J.P.; Torres, Y. On the influence of space holder in the development of porous titanium implants: Mechanical, computational and biological evaluation. Mater. Charact. 2015, 108, 68–78. [Google Scholar] [CrossRef]
- Sheppard, G.; Tassenberg, K.; Nenchev, B.; Strickland, J.; Mesalam, R.; Shepherd, J.; Williams, H. GAKTpore: Stereological Characterisation Methods for Porous Metal Foams in Biomedical Applications. Data 2020. [Google Scholar] [CrossRef]







| Synthetic Image Max Radius (Pixels) | GAKTpore Max Radius (Pixels) | Accuracy (%) | Mean Accuracy (%) |
|---|---|---|---|
| 27 | 26.75 | 99.09 | 99.6 ± 0.2 |
| 37 | 36.77 | 99.39 | |
| 40 | 39.85 | 99.63 | |
| 55 | 54.84 | 99.71 | |
| 63 | 62.84 | 99.75 | |
| 70 | 69.86 | 99.80 | |
| 100 | 99.74 | 99.73 | |
| 200 | 199.50 | 99.75 |
| Shape | Calculated Circularity | GAKTpore Circularity | Accuracy % | Calculated AR | GAKTpore AR | Accuracy % |
|---|---|---|---|---|---|---|
| Circle | 1 | 0.999 | 99.9 | 1 | 1 | 100 |
| Square | 0.785 | 0.790 | 99.4 | 1 | 1 | 100 |
| 1 × 2 Rectangle | 0.698 | 0.702 | 99.5 | 0.5 | 0.5 | 100 |
| Equilateral Triangle | 0.605 | 0.610 | 99.2 | 0.8660 | 0.8696 | 99.6 |
| Hexagon | 0.907 | 0.90981 | 99.7 | 0.8660 | 0.86635 | 99.9 |
| Synthetic Image Shape | Inner Radius | Calculated Waviness | GAKTpore Waviness | Accuracy% |
|---|---|---|---|---|
 | 2 | 0.6094 | 0.6226 | 97.8 |
 | 5 | 0.6023 | 0.5977 | 99.2 |
 | 10 | 0.5851 | 0.5906 | 99.1 |
 | 15 | 0.5647 | 0.5693 | 99.7 |
 | 20 | 0.5430 | 0.5447 | 99.7 |
 | 25 | 0.5212 | 0.5239 | 99.5 |
 | 28 | 0.5084 | 0.5112 | 99.5 |
| Sample | GAKTpore Mean Pore Size (µm) | ImageJ Manual Mean Pore Size (µm) | ||
|---|---|---|---|---|
| Porous Sintered Copper 1 | 5.56 | 1.66 | 5.05 | 2.16 |
| Porous Sintered Copper 2 | 6.68 | 1.74 | 4.83 | 2.08 |
| Porous Sintered Fibrous Platinum | 4.68 | 1.52 | 4.40 | 2.12 |
| Sample | Mean Circ | Mean Wav | Mean AR | |||||
|---|---|---|---|---|---|---|---|---|
| Porous Sintered Copper 1 | 0.85 | 0.16 | 0.94 | 0.09 | 0.67 | 0.17 | 0.14 | 0.07 |
| Porous Sintered Copper 2 | 0.82 | 0.20 | 0.92 | 0.10 | 0.66 | 0.18 | 0.19 | 0.06 |
| Porous Sintered Fibrous Platinum | 0.83 | 0.16 | 0.94 | 0.08 | 0.61 | 0.17 | 0.12 | 0.06 |
| Sample | Mean Pore Size (μm) | Mean Circ | Mean Wav | Mean AR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 mm Porous Copper Disk | 3.58 | 1.17 | 0.62 | 0.25 | 0.33 | 0.18 | 0.61 | 0.16 | 0.10 | 0.06 |
| Collagen Scaffold | 212.60 | 195.76 | 0.67 | 0.25 | 0.56 | 0.22 | 0.58 | 0.17 | 0.16 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheppard, G.; Tassenberg, K.; Nenchev, B.; Strickland, J.; Mesalam, R.; Shepherd, J.; Williams, H. GAKTpore: Stereological Characterisation Methods for Porous Foams in Biomedical Applications. Materials 2021, 14, 1269. https://doi.org/10.3390/ma14051269
Sheppard G, Tassenberg K, Nenchev B, Strickland J, Mesalam R, Shepherd J, Williams H. GAKTpore: Stereological Characterisation Methods for Porous Foams in Biomedical Applications. Materials. 2021; 14(5):1269. https://doi.org/10.3390/ma14051269
Chicago/Turabian StyleSheppard, Gareth, Karl Tassenberg, Bogdan Nenchev, Joel Strickland, Ramy Mesalam, Jennifer Shepherd, and Hugo Williams. 2021. "GAKTpore: Stereological Characterisation Methods for Porous Foams in Biomedical Applications" Materials 14, no. 5: 1269. https://doi.org/10.3390/ma14051269