Enhanced Electrochemical Performance Promoted by Tin in Silica Anode Materials for Stable and High-Capacity Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of SiO2@Pc Composites Material
2.2. Preparation of SiO2@Pc @Sn Composites Material
2.3. Battery Assembly and Electrochemical Measurements
2.4. Characterization
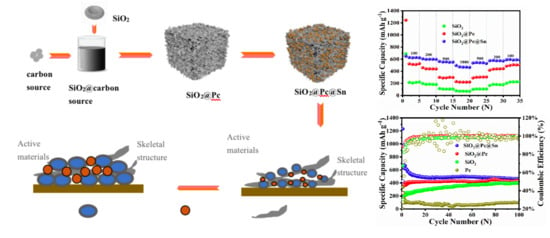
3. Results and Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.-N.; Li, Q.; Ouyang, C.; Yu, X.; Ge, M.; Huang, X.; Hu, E.; Ma, C.; Li, S.; Xiao, R.; et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 2019, 4, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Ji, X.; Fan, X.; Gao, T.; Suo, L.; Wang, F.; Sun, W.; Chen, J.; Chen, L.; Han, F.; et al. Flexible Aqueous Li-Ion Battery with High Energy and Power Densities. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, H.-H.; Ge, M.; Li, L.; Yuan, Y.; Yao, Q.; Chen, J.; Xia, L.; Zheng, J.; Zhao, Y.; et al. Simultaneously Dual Modification of Ni-Rich Layered Oxide Cathode for High-Energy Lithium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1808–1825. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.; Wei, W.; Zhang, L.; Ye, Z.; He, G.; Huang, Y. Nano-ordered structure regulation in delithiated Si an-ode triggered by homogeneous and stable Li-ion diffusion at the interface. Nano Energy 2020, 72, 104651. [Google Scholar] [CrossRef]
- Han, M.; Yu, J. Subnanoscopically and homogeneously dispersed SiOx/C composite spheres for high-performance lithium ion battery anodes. J. Power Sources 2019, 414, 435–443. [Google Scholar] [CrossRef]
- Doughty, D.H.; Crafts, C.C. FreedomCAR Electrical Energy Storage System Abuse Test Manual for Electric and Hybrid Electric Vehicle Applications; Sandia Report SAND2005-3123; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CS, USA, 2006. [Google Scholar]
- Commission NDAR, QC/T-743-2006. Automotive standard of the People’s Republic of China: Lithium Ion Bateries for Electric Vehicles. 2006. Available online: https://max.book118.com/html/2017/0726/124432928.shtm (accessed on 13 February 2021).
- Perner, A.; Vetter, J. Lithium-ion batteries for hybrid electric vehicles. In Advances in Battery Technologies for Electric Vehicles; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 173–190. [Google Scholar]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Whittingham, M.S. ChemInform Abstract: Ultimate Limits to Intercalation Reactions for Lithium Batteries. ChemInform 2015, 114, 11414–11443. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. ChemInform Abstract: Nanomaterials for Energy Conversion and Storage. Cheminform 2013, 44. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-K.; Lee, K.T.; Kim, M.G.; Nazar, L.F.; Cho, J. Recent Progress in Nanostructured Cathode Materials for Lithium Secondary Batteries. Adv. Funct. Mater. 2010, 20, 3818–3834. [Google Scholar] [CrossRef]
- Jia, H.; Gao, P.; Yang, J.; Wang, J.; Nuli, Y.; Yang, Z. Novel Three-Dimensional Mesoporous Silicon for High Power Lithium-Ion Battery Anode Material. Adv. Energy Mater. 2011, 1, 1036–1039. [Google Scholar] [CrossRef]
- Ding, X.; Liu, X.; Huang, Y.; Zhang, X.; Zhao, Q.; Xiang, X.; Li, G.; He, P.; Wen, Z.; Li, J.; et al. Enhanced electrochemical performance promoted by monolayer graphene and void space in silicon composite anode materials. Nano Energy 2016, 27, 647–657. [Google Scholar] [CrossRef]
- Tao, W.; Wang, P.; You, Y.; Park, K.; Wang, C.-Y.; Li, Y.-K.; Cao, F.-F.; Xin, S. Strategies for improving the storage per-formance of silicon-based anodes in lithium-ion batteries. Nano Res. 2019, 12, 1739–1749. [Google Scholar] [CrossRef]
- Ding, X.; Wang, Y. Bilayer-graphene-coated Si nanoparticles as advanced anodes for high-rate lithium-ion bat-teries. Electrochim. Acta 2020, 329, 134975. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef]
- Ding, X.; Wang, H.; Liu, X.; Gao, Z.; Huang, Y.; Lv, D.; He, P.; Huang, Y. Advanced anodes composed of graphene encapsulated nano-silicon in a carbon nanotube network. Rsc Adv. 2017, 7, 15694–15701. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Feng, X.; Chen, Y. High performance lithium battery anode materials by coating SiO2 nanowire arrays with PEO. New J. Chem. 2019, 43, 14609–14615. [Google Scholar] [CrossRef]
- Yi, X.; Yu, W.J.; Tsiamtsouri, M.A.; Zhang, F.; He, W.; Dai, Q.; Hu, S.; Tong, H.; Zheng, J.; Zhang, B. Highly conductive C-Si@G nanocomposite as a high-performance anode material for Li-ion batteries. Electrochim. Acta 2018, 295, 295. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, S.X.; Luo, S.H.; Li, S.H. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Nat. Prod. Rep. 2019, 36, 626–665. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Y.; Xu, C.; Zhu, J.; Wei, X.; Zhou, L.; He, L.; Yang, W.; Mai, L. Ultrafine Nickel-Nanoparticle-Enabled SiO2 Hierarchical Hollow Spheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2018, 28, 1704561. [Google Scholar] [CrossRef]
- Favors, Z.; Wang, W.; Bay, H.H.; George, A.; Ozkan, M.; Ozkan, C.S. Stable cycling of SiO2 nanotubes as high-performance anodes for lithium-ion batteries. Sci. Rep. 2014, 4, 4605. [Google Scholar]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode mate-rial for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895. [Google Scholar] [CrossRef]
- Meng, J.; Cao, Y.; Suo, Y.; Liu, Y.; Zhang, J.; Zheng, X. Facile Fabrication of 3D SiO2@Graphene Aerogel Composites as Anode Material for Lithium Ion Batteries. Electrochim. Acta 2015, 176, 1001–1009. [Google Scholar] [CrossRef]
- Ichikawa, S.; Suda, J.; Sato, T.; Suzuki, Y. Lattice dynamics and temperature dependence of the first-order Raman spectra for? SiO2 crystals. J. Raman Spectrosc. 2003, 34, 135–141. [Google Scholar] [CrossRef]
- Brijesh, K.; Dhanush, P.C.; Vinayraj, S.; Nagaraja, H.S. Monoclinic Wolframite ZnWO4/SiO2 nanocomposite as an anode material for lithium ion battery. Mater. Lett. 2020, 275, 128108. [Google Scholar]
- Di, F.W.; Wang, N.; Li, L.X.; Geng, X.; Yang, H.M.; Zhou, W.M.; Sun, C.G.; An, B.G. Coral-like porous composite material of silicon and carbon synthesized by using diatomite as self-template and precursor with a good performance as an-ode of lithium-ions battery. J. Alloy. Compd. 2021, 854. [Google Scholar] [CrossRef]
- Shen, D.; Huang, C.; Gan, L.; Liu, J.; Gong, Z.; Long, M. Rational Design of Si@SiO2/C Composites Using Sustainable Cellulose as a Carbon Resource for Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 7946–7954. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, X.; Deng, Y.; Wang, L.; Ju, Z. SiO2/N-doped graphene aerogel composite anode for lithium-ion batteries. J. Mater. Sci. 2020, 55, 13023–13035. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, J.; Xue, L.; Huang, T.; Yu, A. Carbon-coated SiO2 nanoparticles as anode material for lithium ion bat-teries. J. Power Sources 2011, 196, 10240–10243. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Li, K.; Zhao, Y.; Zhang, Y.; Gosselink, D.; Chen, P. SiO2/Cu/polyacrylonitrile-C composite as anode mate-rial in lithium ion batteries. J. Power Sources 2013, 240, 659–666. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Ma, M.; Zhou, J.; Liu, Y.; Chen, Y. Preparation of SiO2 nanowire arrays as anode material with en-hanced lithium storage performance. RSC Adv. 2018, 8, 33652–33658. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Cai, L.; Chen, L.; Lin, X.; Fu, R.; Zhang, M.Q.; Wu, D. Silica nanonetwork confined in nitrogen-doped ordered mesoporous carbon framework for high-performance lithium-ion battery anodes. Nanoscale 2015, 7, 3971–3975. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, W.; Yu, P.; Pan, N.; Hu, H.; Zheng, M.; Xiao, Y.; Liu, Y.; Liang, Y. Facile Synthesis of Core-Shell Structured SiO2@Carbon Composite Nanorods for High-Performance Lithium-Ion Batteries. Nanomaterials 2020, 10, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namsar, O.; Autthawong, T.; Laokawee, V.; Boonprachai, R.; Haruta, M.; Kurata, H.; Yu, A.; Chairuangsri, T.; Sarakonsri, T. Improved electrochemical performance of anode materials for high energy density lithium-ion batteries through Sn(SnO2)–SiO2/graphene-based nanocomposites prepared by a facile and low-cost approach. Sustain. Energy Fuels 2020, 4, 4625–4636. [Google Scholar] [CrossRef]
- Tang, C.; Li, N.; Sheng, J.; Zhou, L.; He, L.; Zhu, J.; Li, F.; Liu, Y.; Mai, L. Facile Synthesis of Bi2S3@SiO2 Core-Shell Microwires as High-Performance Anode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, 6110–6115. [Google Scholar] [CrossRef]
- Tu, J.; Yuan, Y.; Zhan, P.; Jiao, H.; Wang, X.; Zhu, H.; Jiao, S. Straightforward Approach toward SiO2 Nanospheres and Their Superior Lithium Storage Performance. J. Phys. Chem. C 2014, 118, 7357–7362. [Google Scholar] [CrossRef]
- Liu, D.D.; Kong, Z.; Liu, X.H.; Fu, A.P.; Wang, Y.Q.; Guo, Y.G.; Guo, P.Z.; Li, H.L.; Zhao, X.S. Spray-Drying induced assembly of skeleton-structured SnO2/Graphene composite spheres as superior anode materials for high-performance lithium-ion batteries. ACS Appl. Mater. Inter. 2018, 10, 2515–2525. [Google Scholar] [CrossRef]
- Xin, F.; Whittingham, M.S. Challenges and Development of Tin-Based Anode with High Volumetric Capacity for Li-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 643–655. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Ren, J.; Jiang, C.; Wan, C. Preparation of Sn/C microsphere composite anode for lithium-ion batteries via carbonthermal reduction. Electrochem. Solid St. 2006, 9, 320–323. [Google Scholar]
- Derrien, G.; Hassoun, J.; Panero, S.; Scrosati, B. Nanostructured Sn–C Composite as an Advanced Anode Material in High-Performance Lithium-Ion Batteries. Adv. Mater. 2007, 19, 2336–2340. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Hou, Z.; Yu, Y.; Di, Q.; Wu, X.; Wei, G.; Quan, Z.; Zhang, J. Monodisperse tin nanoparticles and hollow tin oxide nanospheres as anode materials for high performance lithium ion batteries. Inorg. Chem. Front. 2018, 6, 473–476. [Google Scholar] [CrossRef]
- Tolosa, A.; Widmaier, M.; Krüner, B.; Griffin, J.M.; Presser, V. Continuous silicon oxycarbide fiber mats with tin nanoparticles as high capacity anode for lithium-ion batterie. Sustain. Energy Fuels 2018, 2, 215–228. [Google Scholar]
- Huang, X.; Cui, S.; Chang, J.; Hallac, P.B.; Fell, C.R.; Luo, Y.; Metz, B.; Jiang, J.; Hurley, P.T.; Chen, J. A Hierarchical Tin/Carbon Composite as an Anode for Lithium-Ion Batteries with a Long Cycle Life. Angew. Chem. Int. Ed. 2014, 54, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ben, L.; Zhan, Y.; Huang, X. Nano-Sn embedded in expanded graphite as anode for lithium ion batteries with improved low temperature electrochemical performance. Electrochim. Acta 2016, 187, 186–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Zhang, Y.; Mentbayeva, A.; Wang, X.; Maximov, M.Y.; Liu, B.; Bakenov, Z.; Yin, F. Facile Synthesis of SiO2@C Nanoparticles Anchored on MWNT as High-Performance Anode Materials for Li-ion Batteries. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Liang, Y.; Ma, H.; Peng, Y.; Yang, H. Insights into the conversion behavior of SiO-C hybrid with pre-treated graphite as anodes for Li-ion batteries. Electrochim. Acta 2016, 187, 473–479. [Google Scholar] [CrossRef]
- Ma, B.; Luo, J.; Deng, X.; Wu, Z.; Luo, Z.; Wang, X.; Wang, Y. Hollow Silicon–Tin Nanospheres Encapsulated by N-Doped Carbon as Anode Materials for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2018, 1, 6989–6999. [Google Scholar] [CrossRef]
- Blanco, M.V.; Renman, V.; Vullum-Bruer, F.; Svensson, A.M. Nanostructured diatom earth SiO2 negative electrodes with superior electrochemical performance for lithium ion batteries. RSC Adv. 2020, 10, 33490–33498. [Google Scholar] [CrossRef]
- Ma, B.; Lu, B.; Luo, J.; Deng, X.; Wu, Z.; Wang, X. The hollow mesoporous silicon nanobox dually encapsulated by SnO2/C as anode material of lithium ion battery. Electrochim. Acta 2018, 288, 61–70. [Google Scholar] [CrossRef]
- Gu, Z.; Xia, X.; Liu, C.; Hu, X.; Chen, Y.; Wang, Z.; Liu, H. Yolk structure of porous C/SiO2 /C composites as. anode for lithium-ion batteries with quickly activated SiO2. J. Alloy. Compd. 2018, 757, 265–272. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.; Aken, P.v.A.; Maier, J.; Yu, Y. Dual-Functionalized Double Carbon Shells Coated Silicon Nanoparticles for High Performance Lithium-Ion Batteries. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- He, Y.; Xu, G.; Wang, C.; Xu, L.; Zhang, K. Horsetail-derived Si@N-doped carbon as low-cost and long cycle life anode for Li-ion half/full cells. Electrochim. Acta 2018, 264, 173–182. [Google Scholar] [CrossRef]
- Park, C.-K.; Park, S.-B.; Oh, S.-H.; Jang, H.; Cho, W.-I. Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO 4. Bull. Korean Chem. Soc. 2011, 32, 836–840. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Liang, D.; Zhao, H. Enhanced Electrochemical Performance Promoted by Tin in Silica Anode Materials for Stable and High-Capacity Lithium-Ion Batteries. Materials 2021, 14, 1071. https://doi.org/10.3390/ma14051071
Ding X, Liang D, Zhao H. Enhanced Electrochemical Performance Promoted by Tin in Silica Anode Materials for Stable and High-Capacity Lithium-Ion Batteries. Materials. 2021; 14(5):1071. https://doi.org/10.3390/ma14051071
Chicago/Turabian StyleDing, Xuli, Daowei Liang, and Hongda Zhao. 2021. "Enhanced Electrochemical Performance Promoted by Tin in Silica Anode Materials for Stable and High-Capacity Lithium-Ion Batteries" Materials 14, no. 5: 1071. https://doi.org/10.3390/ma14051071