Tool for Designing Breakthrough Discovery in Materials Science
Abstract
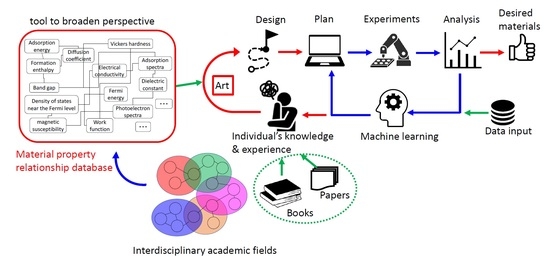
:1. Introduction
2. Examples of Knowledge Utilization
2.1. Substrate for the Growth of Ultra-Thin Atomically Flat Epitaxial Alumina Film
2.2. Thermoelectric Materials
2.3. Prediction of Work Function from Vickers Hardness
3. Relationship between Material Properties
4. Computer Systems
5. Conclusions
6. Patents
- (1)
- Search System, Search Method, and Physical Property Database Management Device, Japanese Patent #6719748, US Patent allowed (publication # 2019/0139279).
- (2)
- Search System, Search Device, and Search Method, Japanese Patent # 6876344, US Patent # 11163829
- (3)
- Search System, and Search Method, PCT/JP2019/028188.
- (4)
- Search System, and Search Method, PCT/JP2019/030108.
- (5)
- Search System, and Search Method, Japanese Patent publication #2021-012502.
Funding
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Cawse, J.N. (Ed.) Experimental Design for Combinatorial and High Throughput Materials Development; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Potyrailo, R.A.; Maier, W.F. (Eds.) Combinatorial and High-Throughput Discovery and Optimization of Catalysts and Materials; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Maier, W.F.; Stwe, K.; Sieg, S. Combinatorial and High-Throughput Materials Science. Angew. Chem. Int. Ed. 2007, 46, 6016–6067. [Google Scholar] [CrossRef]
- Laboratory Robotics. Available online: https://en.wikipedia.org/wiki/Laboratory_robotics (accessed on 12 October 2021).
- Researchers Build Robot Scientist That Has Already Discovered a New Catalyst. Available online: https://phys.org/news/2020-07-robot-scientist-catalyst.html (accessed on 29 October 2021).
- Burger, B.; Maffettone, P.M.; Gusev, V.V.; Aitchison, C.M.; Bai, Y.; Wang, X.; Li, X.; Alston, B.M.; Li, B.; Clowes, R.; et al. A mobile robotic chemist. Nature 2020, 583, 237–241. [Google Scholar] [CrossRef]
- Isayev, O.; Tropsha, A.; Curtarolo, S. (Eds.) Materials Informatics: Methods, Tools, and Applications, 1st ed.; Wiley: Weinheim, Germany, 2019. [Google Scholar]
- Rajan, K. (Ed.) Informatics for Materials Science and Engineering: Data-Driven Discovery for Accelerated Experimentation and Application; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lookman, T.; Alexander, F.J.; Rajan, K. (Eds.) Information Science for Materials Discovery and Design, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Lopez-Bezanilla, A.; Littlewood, P.B. Growing field of materials informatics: Databases and artificial intelligence. MRS Commun. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Rajan, K. Materials Informatics: The Materials “Gene” and Big Data. Annu. Rev. Mater. Res. 2015, 45, 153–169. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, S.; Stevenson, K.J. Influence of Nitrogen Doping on Oxygen Reduction Electrocatalysis at Carbon Nanofiber Electrodes. J. Phys. Chem. B 2005, 109, 4707–4716. [Google Scholar] [CrossRef]
- Ozaki, J.; Tanifuji, S.; Kimura, N.; Furuichi, A.; Oya, A. Enhancement of oxygen reduction activity by carbonization of furan resin in the presence of phthalocyanines. Carbon 2006, 44, 1324–1326. [Google Scholar] [CrossRef]
- Yoshitake, M. Materials Curation: An Innovative Method for Material Search by Exploring Material Information from a Comprehensive Viewpoint. Kinou Zair. 2013, 33, 48–55. (In Japanese) [Google Scholar]
- Yoshitake, M. Materials Curation: A Tool for Drawing Material Design Principles. Int. J. Sci. Res. 2015, 4, 571–579. [Google Scholar]
- Yoshitake, M.; Kuwajima, I.; Yagyu, S.; Chikyow, T. System for Searching Relationship among Physical Properties for Materials CurationTM. Vac. Surf. Sci. 2018, 61, 200–205. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Yoshitake, M. Utilizing Knowledge on Scientific Principles on Material Properties for Materials R&D. J. Surf. Anal. 2019, 26, 134–135. (In Japanese) [Google Scholar] [CrossRef]
- Yoshitake, M. Materials Curation®: Material Search Using Scientific Principles from a Comprehensive Viewpoint. J. Comput. Chem. Jpn. 2020, 19, 36–42. (In Japanese) [Google Scholar] [CrossRef]
- Jaeger, R.M.; Kuhlenbech, H.; Freund, H.-J.; Wuttig, M.; Hoffmann, W.; Franchy, R.; Ibach, H. Formation of a well-ordered aluminium oxide overlayer by oxidation of NiAl (110). Surf. Sci. 1991, 259, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.J.; Goodman, D.W. Epitaxial growth of ultrathin Al2O3 films on Ta (110). Surf. Sci. 1994, 312, L767–L773. [Google Scholar] [CrossRef]
- Wu, M.C.; Goodman, D.W. Particulate Cu on Ordered Al2O3: Reactions with Nitric Oxide and Carbon Monoxide. J. Phys. Chem. 1994, 98, 9874–9881. [Google Scholar] [CrossRef]
- Graupner, H.; Hammer, L.; Heinz, K.; Zehner, D.M. Oxidation of low-index FeAl surfaces. Surf. Sci. 1997, 380, 335–351. [Google Scholar] [CrossRef]
- Herman, M.A.; Sitter, H. Molecular Beam Epitaxy, 2nd rev. and updated ed.; Springer: Berlin/Heidelberg, Germany, 1996; p. 231. [Google Scholar]
- Lykhach, Y.; Moroz, V.; Yoshitake, M. Formation of epitaxial Al2O3/NiAl (1 1 0) films: Aluminium deposition. Appl. Surf. Sci. 2005, 241, 250–255. [Google Scholar] [CrossRef]
- Yoshitake, M.; Lykhach, Y. Flexible structure of alumina at the interface observed by RHEED. In Proceedings of the 9th International Conference on Atomically Controlled Surfaces, Interfaces and Nanostructure, Tokyo, Japan, 11–15 November 2007; p. 335, Presented at ACSIN-9, PS2-23. [Google Scholar]
- Yamauchi, Y.; Yoshitake, M.; Song, W. Morphology and Thickness of Ultra-Thin Epitaxial Al2O3 Film on Cu-9%Al (1 1 1). Jpn. J. Appl. Phys. 2003, 42, 4721–4724. [Google Scholar] [CrossRef]
- Nemsak, S.; Yoshitake, M.; Masek, K. Ultra-thin oxide layer formation on Cu–9% Al (1 1 1) surface and Pd growth studied using reflection high energy electron diffraction and Auger electron spectroscopy. Surf. Sci. 2006, 600, 4357–4360. [Google Scholar] [CrossRef]
- Yoshitake, M.; Nagata, T.; Song, W. Electrical properties and stability of an epitaxial alumina film formed on Cu-9 at. % Al (111). J. Vac. Sci. Technol. A 2012, 30, 021509. [Google Scholar] [CrossRef]
- Yoshitake, M. Materials Curation: Case study #2. In Proceedings of the JSAP Autumn Meeting, Hokkaido University, Sapporo, Hokkaido, Japan, 17–20 September 2014. [Google Scholar]
- Yoshitake, M. Application of Materials Curation to Thermoelectric Materials. In Thermoelectric Materials; NIMS Research Report; NIMS: Tsukuba, Japan, 2015; pp. 181–200. ISBN 978-4-990056360. [Google Scholar]
- Nolas, G.S.; Sharp, J.; Goldsmid, H.J. Thermoelectrics; Springer: New York, NY, USA, 2001; p. 56. [Google Scholar]
- Thermoelectric Properties of Materials. Available online: http://thermoelectrics.matsci.northwestern.edu/thermoelectrics/index.html (accessed on 29 October 2021).
- Gruzalski, G.R.; Lui, S.-C.; Zehner, D.M. Work-function changes accompanying changes in composition of (100) surfaces of HfCx and TaCx. Surf. Sci. Lett. 1990, 239, L517–L520. [Google Scholar] [CrossRef]
- Price, D.L.; Cooper, B.R.; Wills, J.M. Effect of carbon vacancies on carbide work functions. Phys. Rev. B 1993, 48, 15311–15315. [Google Scholar] [CrossRef]
- Yoshitake, M. Work Function and Band Alignment of Electrode Materials; Springer: Tokyo, Japan, 2021. [Google Scholar] [CrossRef]
- Yoshitake, M. Generic trend of work functions in transition-metal carbides and nitrides, J. Vac. Sci. Technol. A 2014, 32, 061403. [Google Scholar] [CrossRef]
- Search System, Search Method, and Physical Property Database Management Device. Japanese Patent #6,719,748, 19 June 2020.
- Search System, Search Device, and Search Method. Japanese Patent # 6,876,344, 28 April 2021.
- Bondy, J.A.; Murty, U.S.R. Graph Theory with Applications; Elsevier Science Ltd.: North-Holland, Netherlands, 1976. [Google Scholar]
- Yoshitake, M.; Kuwajima, I.; Yagyu, S.; Chikyow, T. Development of Search System of Material Properties Relation Diagram. In Proceedings of the JSAP Spring Meeting, Pacifico Yokohama, Japan, 14–17 March 2017. [Google Scholar]
- Yoshitake, M.; Sato, F.; Kawano, H. Developing a Materials Curation® Support System. J. Surf. Anal. 2020, 27, 22–33. [Google Scholar] [CrossRef]
- Zarrinfar, N.; Shipway, P.H.; Kennedy, A.R.; Saidi, A. Carbide stoichiometry in TiCx and Cu–TiCx produced by self-propagating high-temperature synthesis. Scr. Mater. 2002, 46, 121–126. [Google Scholar] [CrossRef]
- Nachiappan, C.; Rangaraj, L.; Divakar, C.; Jayaram, V. Synthesis and densification of monolithic zirconium carbide by reactive hot pressing. J. Am. Ceram. Soc. 2010, 93, 1341–1346. [Google Scholar] [CrossRef]
- Lipatnikov, V.N.; Gusev, A.I.; Ettmayer, P.; Lengauer, W. Phase transformations in non- stoichiometric vanadium carbide. J. Phys. Condens. Matter. 1999, 11, 163–184. [Google Scholar] [CrossRef]
- Bowman, A.L. The variation of lattice parameter with carbon content of Tantalum carbide. J. Phys. Chem. 1961, 65, 1596–1598. [Google Scholar] [CrossRef]
- Massalski, T.B. (Ed.) Binary Alloy Phase Diagrams; ASM International: Almere, Netherlands, 1990. [Google Scholar]












| Book Title | Author(s) | Publisher | Year |
|---|---|---|---|
| Fundamentals of Materials Science | Eric J. Mittemeijer | Springer | 2011 |
| Understanding Materials Science | Rolf E. Hummel | Springer | 2004 |
| Materials Handbook | François Cardarelli | Springer | 2018 |
| The Chemical Bond I–III | D. Michael P. Mingos, ed. | Springer | 2016 |
| Ceramic Materials: Science and Engineering | C.Barry Carter, M. Grant Norton | Springer | 2013 |
| Electrochemistry for Materials Science | Walfried Plieth | Elsevier | 2008 |
| Solid State Electrochemistry I: Fundamentals, Materials and their Applications | Vladislav V. Kharton | WILEY | 2009 |
| Electronic Properties of Materials | E Hummel | Springer | 2011 |
| Physics of Semiconductor Devices | Simon M. Sze, Kwok K. Ng | WILEY | 2006 |
| Principles of Surface Physics | Friedhelm Bechstedt | Springer | 2003 |
| Physics of Surfaces and Interfaces | Harald Ibach | Springer | 2006 |
| Solid Surface Physics | Heribert Wagner | Springer | 1979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshitake, M. Tool for Designing Breakthrough Discovery in Materials Science. Materials 2021, 14, 6946. https://doi.org/10.3390/ma14226946
Yoshitake M. Tool for Designing Breakthrough Discovery in Materials Science. Materials. 2021; 14(22):6946. https://doi.org/10.3390/ma14226946
Chicago/Turabian StyleYoshitake, Michiko. 2021. "Tool for Designing Breakthrough Discovery in Materials Science" Materials 14, no. 22: 6946. https://doi.org/10.3390/ma14226946